This fall, we will have vaccines for all three fall respiratory viruses: flu, RSV, and Covid-19. It’s getting hard to keep track of all of them. So here are the what, who, and when for each, informed by the most up-to-date science.
There are some nuances for those looking for ultimate protection, but in the end, the best vaccine is the one you get!
You can find a one-page, concise, downloadable PDF summary at the bottom of this post. (Many physician offices and health departments have found it helpful to print.)
Seasonal influenza (flu)
What: The vaccine covers three strains of seasonal flu and is offered by four pharmaceutical companies. Selecting vaccine strains for rapidly changing viruses, like flu or Covid-19, is both an art and a science, so the vaccine formula doesn’t always align perfectly with the circulating virus. However, this year’s composition was an excellent match to the flu strains in Australia (which is a good predictor of the upcoming Northern Hemisphere season). Flu vaccines reduce the risk of needing to go to the doctor by 40% to 60%.
Who: Everyone 6 months and older. Special formulations provide added protection for older adults. Children should get two shots one month apart during their first flu season.
When: Protection wanes throughout the season, so October is the best time to get vaccinated. The complete list of timing recommendations for specific populations (pregnant people, older adults, young children) is available here.
Which one: The vaccines are all very similar, and you won’t gain much from shopping around. The nasal spray flu vaccine may work a bit better in children. A recent study suggested that the adjuvanted flu vaccine (Fluad) might work better in older adults.
COVID-19 vaccine
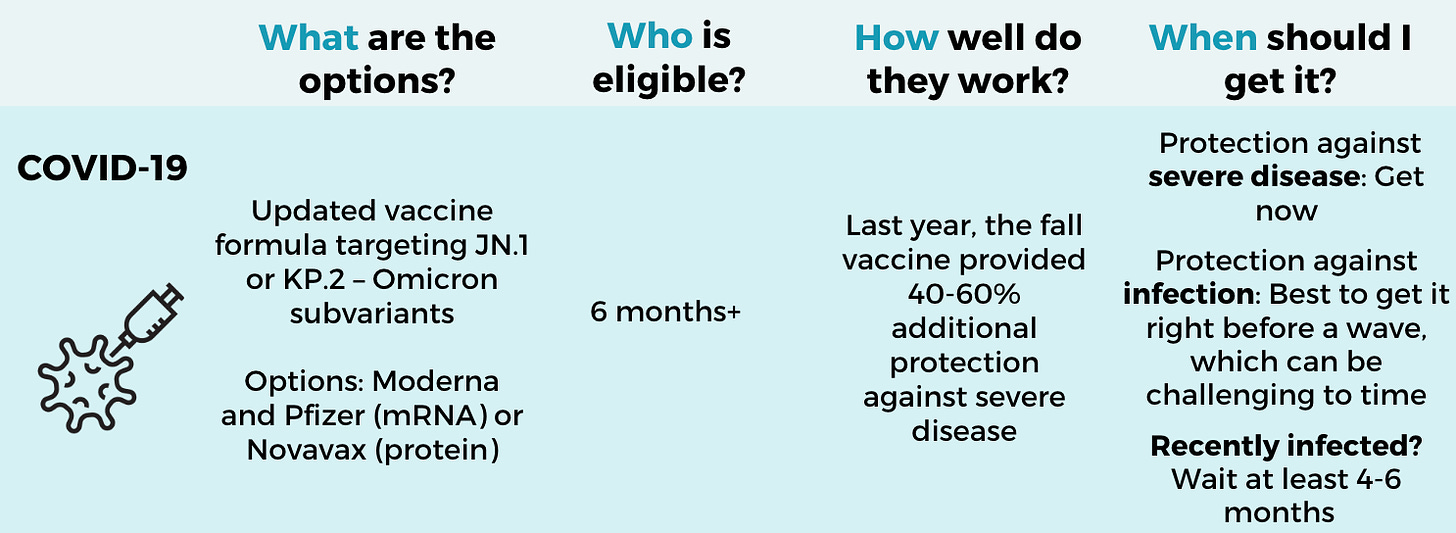
What: The fall Covid-19 vaccines have an updated formula targeting JN.1 (Novavax) or KP.2 (Pfizer or Moderna). We don’t know their effectiveness in humans yet, but updated vaccines provided ~60% additional protection last fall.
This season is more complicated because there are two slightly different Covid-19 choices. There are pros and cons to each:
mRNA vaccines (Pfizer or Moderna) are more up-to-date, targeting the latest Omicron subvariants, and are presumably more effective against infection (in the short term). Both manufacturers made a JN.1 vaccine but found that the KP.2 was better in inducing antibody responses against current variants. The Pfizer vaccine is probably better than Moderna for those at higher risk of myocarditis (i.e., younger men).
The traditional protein vaccine (Novavax) cannot be updated as quickly, so it had to go with the older subvariant version. Novavax’s data suggest that this is probably okay, as even this older variant version gave good responses against current variants. For some (including me!), the side effects of mRNA vaccines can be intense. I’ll be getting Novavax for this reason.
We don’t know if Novavax performs better (or worse) than mRNA vaccines. The very few studies we do have come to different conclusions.
Who: Everyone 6 months and older.
When: These are expected to be available soon. Word on the street is the mRNA vaccines may even be approved by FDA this week. Novavax should closely follow. However, “now” isn’t necessarily when you should get them:
If you were recently infected, wait 4-6 months. It doesn’t hurt if you get it earlier, but some research shows that waiting allows our antibody factories to update more effectively.
If you were not recently infected, the timing is a tough call. Either get it now—we are in the middle of a huge infection wave—or wait to increase protection against the winter wave (which may be closer to November). I will be getting mine when it becomes available.
RSV vaccine for older adults
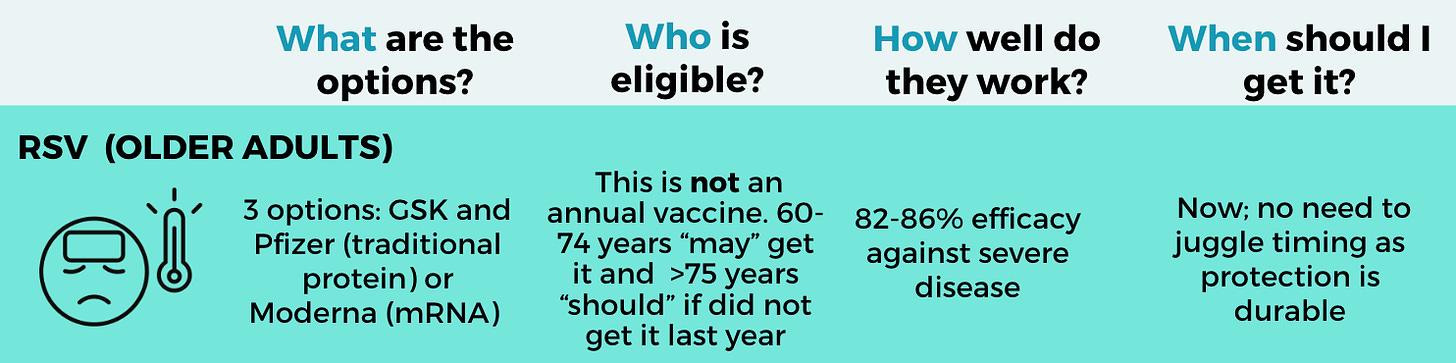
What: This season, there are three RSV vaccines: GSK, Pfizer, and Moderna. There are pros and cons to each:
GSK and Pfizer use traditional biotechnology (protein-based), which was available last year, so we have lots of “real world” data confirming safety and effectiveness. There is a small (but real) risk of Guillain-Barre syndrome—the risk is about the same as with flu vaccines.
Moderna is an mRNA vaccine expected to become available this season. It did not have a Guillain-Barre syndrome safety signal, but protection wanes more quickly.
Who: This is not an annual vaccine—If you got one last year, you do not need one this year. Studies showed getting a second dose didn’t meaningfully enhance protection. People ages 60 and older “may” get the vaccine. Those over 75 years “should.”
When: RSV vaccines show some initial waning in the first few weeks after vaccination but then stabilize at a high level of protection for more than one year, so getting one now should protect you throughout the entire season (and then some).
RSV vaccine for pregnancy
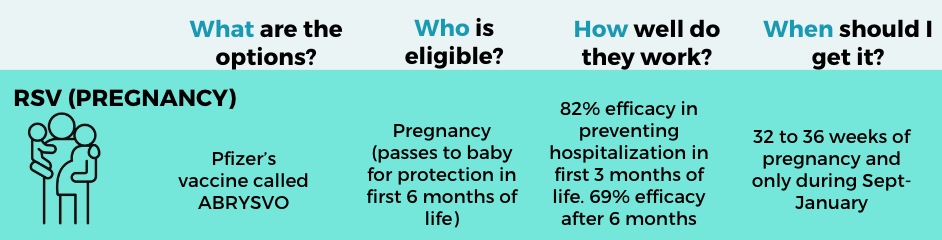
What: One vaccine is available: Pfizer. Protection is passed from the mother to the baby so that the baby is protected in the first 6 months of life, which is the riskiest time for severe RSV. Thousands of pregnant women got it last year, confirming the safety and high effectiveness (70-85%).
When: During 32-36 weeks of pregnancy from September to January. This vaccine can be given simultaneously with other routine vaccines for pregnancy (Tdap, Covid-19, and influenza). Some data shows that getting an RSV vaccine at the same time as Tdap may reduce the antibody response to pertussis. So, it may be worth considering getting the Tdap vaccine a few weeks before, but there is no formal recommendation.
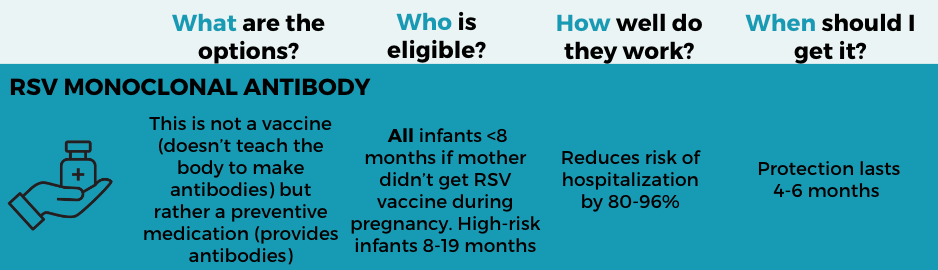
RSV monoclonal antibody for infants
What: Beyfortus (nirsevimab) is not a vaccine (i.e., it doesn’t teach the body to make an immune response)—it is a preventive medication (providing antibodies directly and proactively). Last year’s real-world data showed that severe RSV in infants was drastically reduced; one study achieved 98% effectiveness. This is a game-changer for babies!
Who: All infants under 8 months should get it for their first RSV season, unless the mother received the RSV vaccine during pregnancy. High-risk children between 8 months to 19 months should also get it. If the mother got the RSV vaccine during pregnancy, getting a monoclonal antibody is not recommended unless the infant is at very high risk.
When: Generally, as close to RSV season as possible (protection holds up for at least 5 months). Last season, there was a supply issue, but it should be ironed out this year.
Bottom line
Get protected! It is one of the best things you can do this fall and winter to stay healthy and minimize disruption. As always, for specific questions or guidance, be sure to talk with your healthcare provider.
Below is a PDF version of the summary for paid subscribers. Feel free to download, print, share, or doodle on.
Love, YLE