Today the ACIP met to discuss the safety and effectiveness of the adolescent Pfizer vaccine. This is the final step in approving the vaccine for mass distribution. Here are the slides. And here are your cliff notes…
Bottom Line: Things are looking good. Well, not just good, they are looking great. This trial was as clean as it can get. There is nothing (big) we didn't expect. This vaccine is incredibly safe. And works incredibly well. I strongly support the use of this vaccine among those aged 12+. And this is urgent. If I had an adolescent, I would get them the vaccine as soon as possible.
Study Overview
This was considered a Phase 2/3 clinical trial. This means the study was designed to assess immunogenicity (how well the vaccine works at developing antibodies, which is Phase 2) and efficacy (how well the vaccine prevents COVID19, which is Phase 3).
Two age groups were in this study:
12-15 year old cohort: 1,131 in vaccine group and 1,129 in placebo group
16-25 year old cohort: 1,867 in vaccine group and 1,903 in placebo group
Doses were given 3 weeks apart, same dosage as the adults, and saline placebo
Every participant had at least 1 month of follow-up data. 4.3% of subjects had more than 3 months of follow-up time for this data
Safety
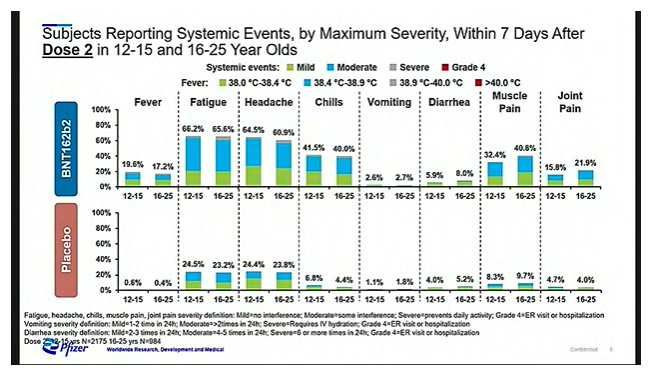
Mostly mild-to-moderate side effects were reported. Overall, adolescents showed a similar side effect pattern to adults:
Vaccine group reported higher rates of fever, fatigue, headaches, chills, diarrhea, muscle pain, and joint pain than the placebo group
Worse side effects with the second dose compared to the first
Adverse events: 6.0% in vaccine group and 5.9% in the placebo group
There was a small numerical imbalance of mental illness among the vaccine group (5 cases) compared to the placebo group (0 cases). The FDA and CDC looked into this very carefully to ensure SSRI’s don’t worsen depression or suicide ideation among vaccinated adolescents. (This is probably why it took so long for EUA approval). After intensive review, there was no casual link between psychological events and the vaccine
An unsolicited (i.e. not expected) adverse event that came up more in the vaccine group was swollen lymph nodes (lymphadenopathy). 9 in the vaccine group vs. 2 in the placebo group. This could be related to the vaccine. All cases resolved within 1 week of starting.
Serious adverse events: 5 in the vaccine group (0.4%) and 2 in the placebo group (0.2%). None of these adverse events were connected to the vaccine.
1 pregnancy after vaccination in the 16-25 year old cohort. 0 pregnancies in the 12-15 year old cohort.
No cases of Bells Palsy, anaphylaxis, blood clots, MIS-C, or deaths were reported by any trial participants (vaccine or placebo)
Immunogenicity
One main purpose of this trial was to see if vaccines worked (i.e. create neutralizing antibodies) as well in adolescents as in young adults. In order to do this, participants were randomly selected to give blood.
The immune response was actually better among adolescents (12-15 years) than compared to young adults (16-25 years old). Which is fantastic.
Efficacy
In the vaccine group, there were 3 COVID19 cases after the 1st dose
After the 2nd dose, there was 100% efficacy (there were 16 cases of COVID among the placebo and 0 among the vaccinated)
There were no severe cases of COVID19 during this study
YES, COVID-19 disease among adolescents is a public health problem
1.5 million cases of COVID among adolescents throughout the pandemic. After adjusting for underreporting, 22.2 million children (5-17 years of age) has had COVID19. This attributes to 19% of the cases in the general population.
Adolescents have the highest rate of infection and symptomatic infection (compared to adults and children), but hospitalizations are lower than adults
127 COVID19 adolescent deaths since the beginning of the pandemic. Although this seems low, death among adolescents is low overall. This ranks COVID19 as a top 10 cause of death in the United States for adolescents.
3,742 MIS-C cases since the pandemic
21% were among adolescents
Severity of MIS-C (like heart function and ICU admission) is worse for adolescents compared to younger children
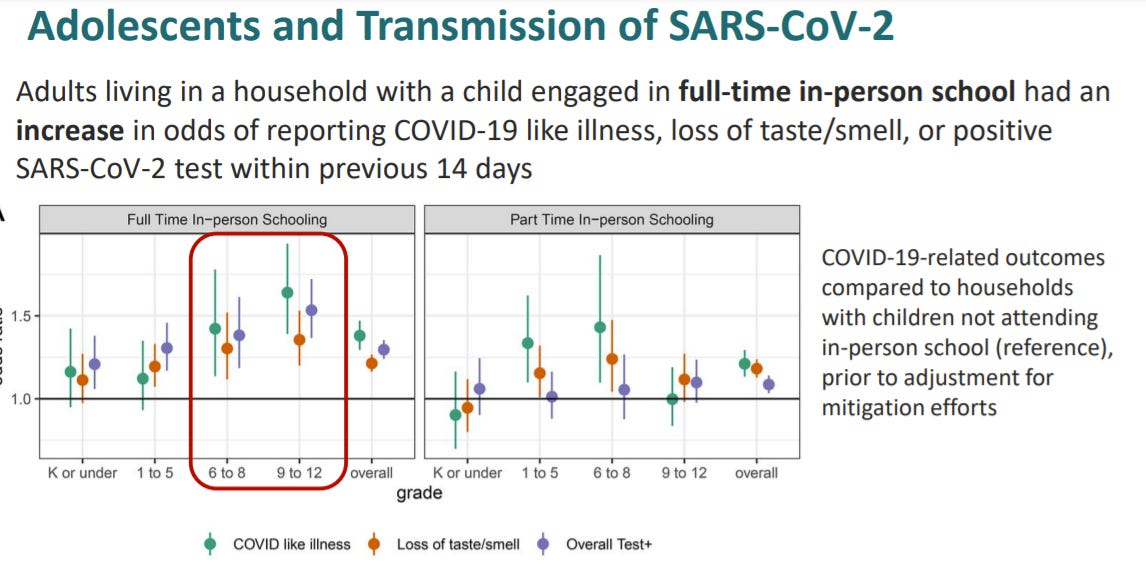
Transmission is higher in high school settings than elementary school settings
Living with a child engaged in-person school increases the odds of COVID19 for the household compared to children not in attendance of in-person school. In other words, vaccinations are incredibly important with the opening of schools.
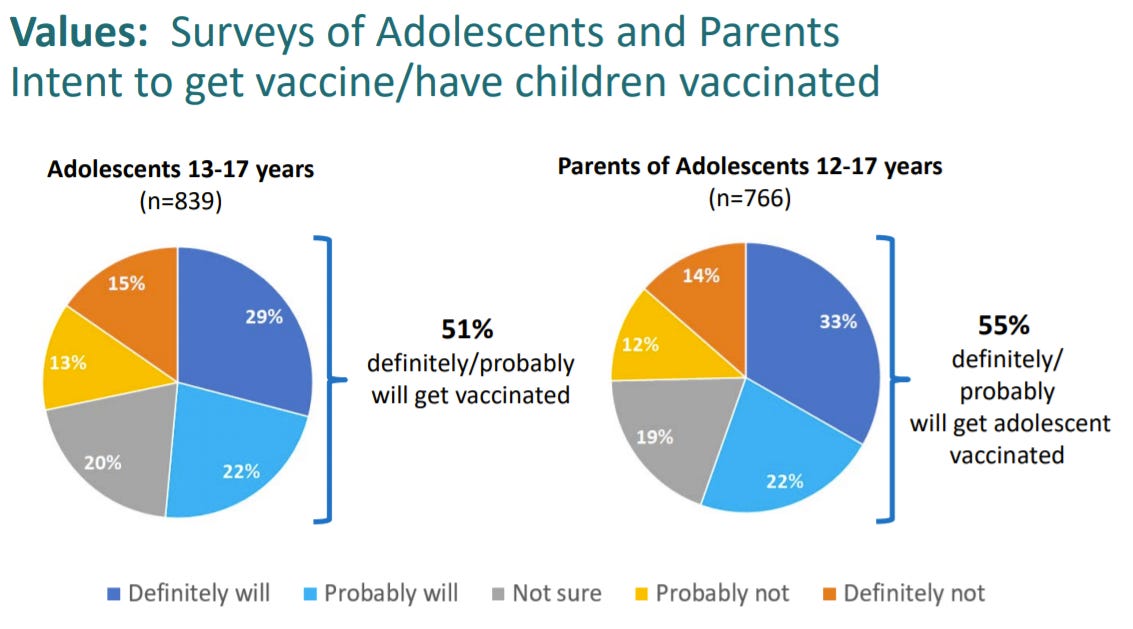
Vaccine hesitancy among adolescents and parents
The good news is that 50% of adolescents/parents want to get the vaccine. The bad news is that 50% of adolescents/parents want to get the vaccine. We need this to increase.
Co-administration
There was a LOT of discussion around how COVID19 vaccines should be administered with other childhood vaccines. Up until now, it’s recommended that people wait 14 days after COVID19 vaccine for other vaccines. This CDC recommendation was originally made out an abundance of caution; it was not evidence-based.
Some members strongly voiced that we need data in order to remove this requirement. Vaccines could interact with each other, particularly around vaccine effectiveness and side effects. We need to follow this closely.
Other members reminded us that, unfortunately though, this information was not systematically collected or tested in clinical trials so it would take a while to assess. And, we are in the middle of an unprecedented pandemic. And, it’s hard to get adolescents back into a visit multiple times and we don’t want adolescents to get behind on their vaccine schedule.
Vote
The external committee then was asked to discuss and vote: “The Pfizer vaccine is recommended for persons 12-15 years of age in the U.S. under the FDA Emergency Use Authorization.”
Yes- 14 votes
No- 0 votes
Recusal-1 vote
Motion passes. Adolescents can now go get their vaccine. Cheers!
Love, YLE




My shot phobic 13 year old is actually looking forward to it. He wants to get back to game nights 😊❤️
"•Living with a child engaged in-person school increases the odds of COVID19 for the household compared to children not in attendance of in-person school. In other words, vaccinations are incredibly important with the opening of schools."
And this is why our child will not be returning to school until vaccines are made available for those in the pediatric population under 12.