A fourth mRNA dose has been underway in Israel for a few months now. The U.K. recently started a fourth dose for people over 75 years old and coined it “spring boosters.” In Germany, those over 70 years old are eligible for a fourth shot of the mRNA series.
In the U.S., Pfizer announced they were seeking an EUA for a fourth dosage for people 65 years or older. Moderna is also seeking approval for a fourth dose for everyone. (J&J folks are still kept in the dark; see my note at the end of this post.) On April 6, the FDA external advisory group, VRBPAC, will meet to discuss considerations for future doses. No votes will be taken and no individual vaccine applications will be considered.
So, this begs some questions:
How well are third doses working?
Is the fourth dose effective in Israel?
And, most importantly, what’s our long-term game plan?
Here are some answers.
A third dose is critical
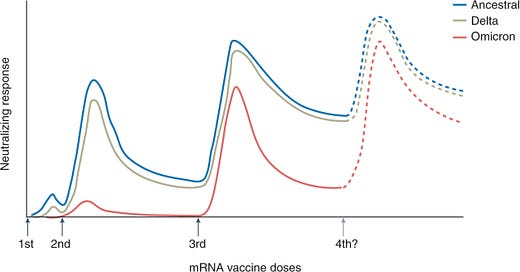
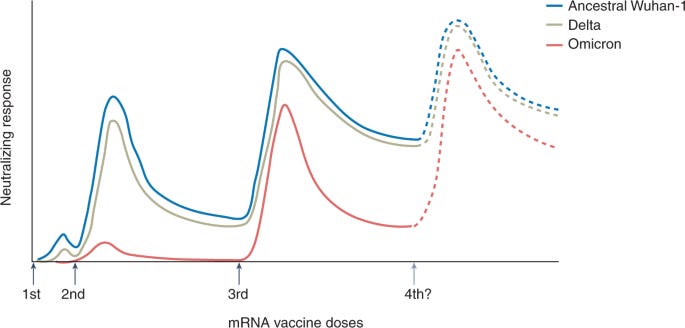
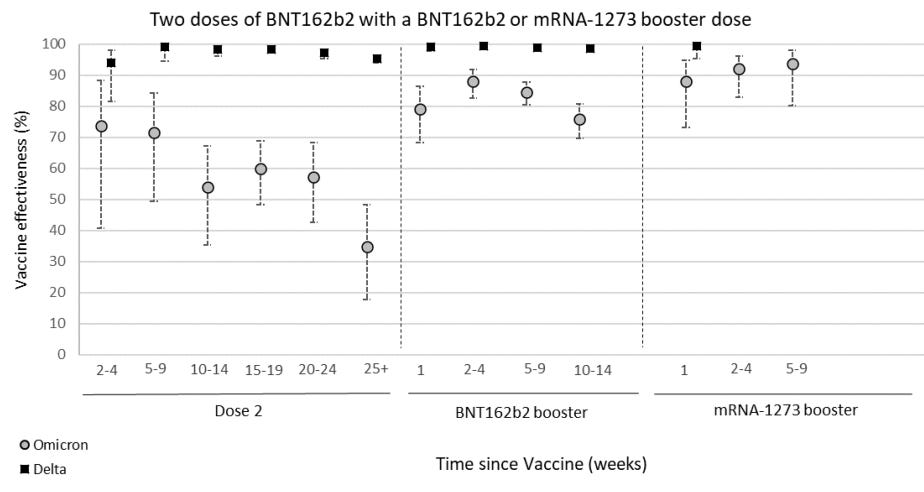
There is no question that a third dose of the mRNA series is critical. The combination of serious immune evasion from Omicron coupled with waning protection over time really impacted vaccine effectiveness. As a recent Nature article pointed out, a third dose against Omicron is about equal to a second dose against Delta. Omicron really made us step up our game.
How are boosters holding up over time?
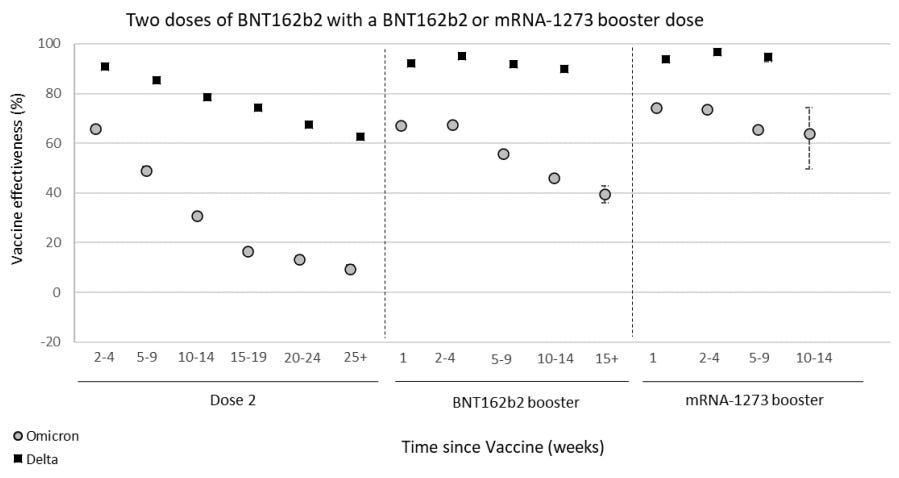
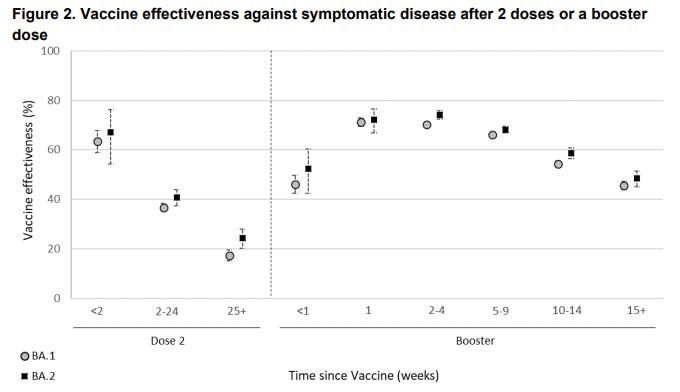
The best real-time data to follow on vaccine effectiveness (VE) over time is in the U.K. The U.K. Health Security Agency currently has follow-up data for 15 weeks after the third dose. In their latest report (published yesterday), it’s clear that VE against infection is significantly waning over time after a third dose. VE continues to be the same for BA.1 compared to BA.2.
VE against hospitalization is holding up much better. But even that is slightly decreasing over time. Moderna seems to be holding up better, but in the U.K. not many get the Moderna vaccine, so there is great uncertainty around these statistics and not much follow-up time.
While this data is powerful, 15 weeks of follow-up data isn’t very helpful in the U.S. because many Americans are coming up on six months (~24 weeks) after their third dose.
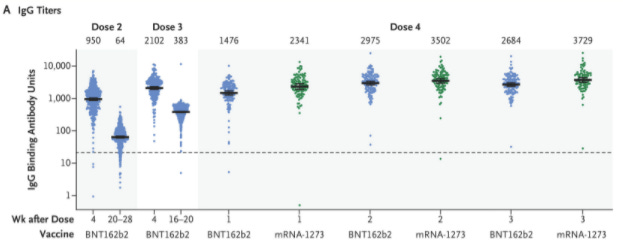
Thankfully, a recent study assessed the durability of a Moderna third dose after 6 months. They found a 6.3-fold decrease in neutralizing antibodies. Importantly there was still detectable protection. (This was a much different story than for the second dose which showed a 7.8-fold decrease and very little detectable protection after 7 months.) Although the decline after a third dose was slower, there was still a meaningful decline. In other words, the durability trend of the third shot is not ideal.
The CDC also published data on VE against Omicron over time in the U.S. They found significantly waning protection against emergency department/urgent care visits. VE against hospitalization was decreasing a little but largely held up 5 months after the third dose.

Older adults
The aforementioned studies pooled everyone. But we know VE among older adults is suboptimal. And, because of this, we’ve seen severe breakthrough infections at a much higher rate among 65+ year olds. The Kaiser Family Foundation found that among severe breakthrough infections, 69% were among older adults. So it’s imperative that we track VE among this group specifically.
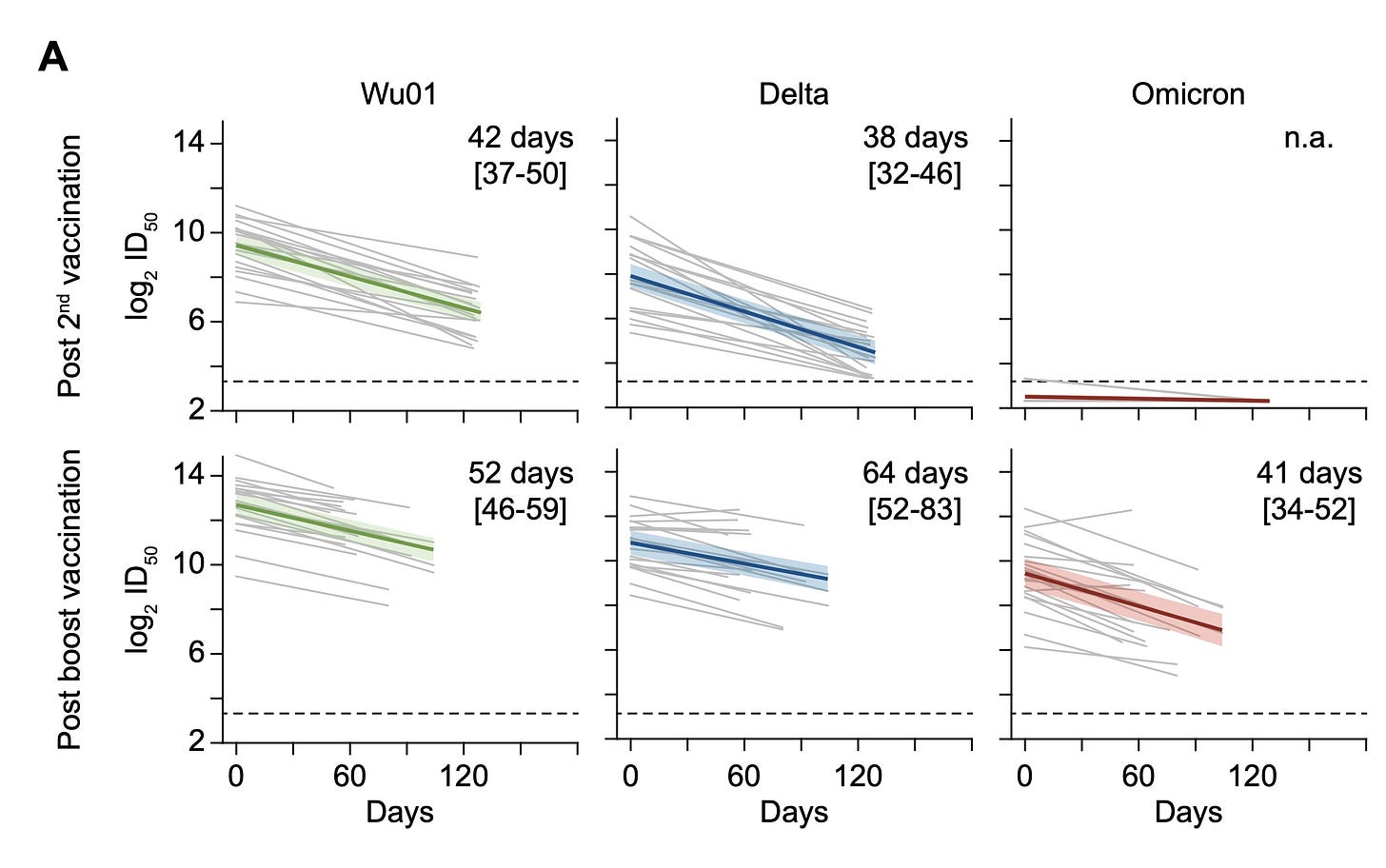
The Lancet recently published a small study that assessed the durability of a third dose among people aged 76 to 96 years old. Study participants were followed for 10 months after their second Pfizer dose and 4.5 months after their third Pfizer dose. They found a third dose was crucial for VE. They also found the third dose half life was 52 days for the Wuhan variant, 64 days for Delta, and 41 days for Omicron. So boosters are waning faster for Omicron. But are largely still protective 4.5 months after the third dose for older adults.
In yesterday’s U.K. report, VE against hospitalization among 65+ year olds was also reported. They assessed VE using three different definitions of hospitalization. In all, the story was the same: Vaccine protection against severe disease is waning slightly among 65+ year olds but not by much.

Value of a fourth dose?
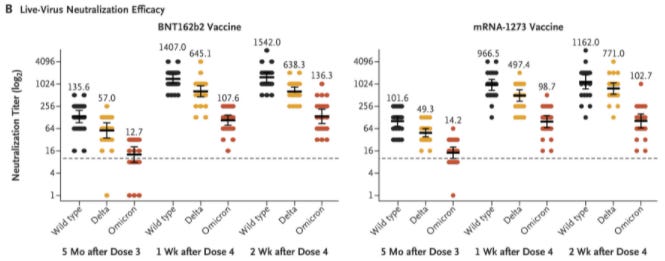
Now that Israel has been delivering a fourth dose for some time, we can start assessing the efficacy. The results are absolutely fascinating.
In a preprint, scientists assessed the rates of infection and severe illness after a fourth dose among 1,138,681 Israelis aged 60+ years. They found that after a fourth dose, the rate of infection was 2 times lower and the rate of severe disease was 4 times lower. A fourth booster had a meaningful impact for older adults.
Another published study assessed the effectiveness of a fourth dose among young healthcare workers. They confirmed that antibodies were significantly reduced 5 months after the third dose. But a fourth dose pushed these back up.
Importantly, this study found that immunogenicity maxes out at 3 doses. In other words, there was no meaningful increase in the quality of the immune system after a fourth dose among the young population. They also assessed the efficacy of the fourth dose compared to the third dose, and the added benefit was not statistically significant. In other words, the efficacy of a fourth dose was no different from the efficacy of a third dose.
Should we get a fourth dose?
This is an incredibly difficult question, and the FDA meeting will be important to follow. They will discuss some key questions:
What is the purpose of vaccines: to prevent infection or to prevent severe disease? Vaccines are still doing a great job at the latter.
What are the costs/benefits of vaccinating now compared to vaccinating in a few months prior to the fall/winter? In other words, should we wait a few months on the fourth dose and focus efforts on getting everyone the third dose? (Only 44% of Americans have the booster.)
Are there scientific results not publicly available, like the effectiveness of variant-specific vaccines? It’s about time we see these.
The FDA may approve a fourth dose for 65+ in the coming weeks. I would be very surprised if they approved another dosage for everyone.
But a fourth dose of the same thing would only be a short-term solution. It’s not practical or sustainable to roll-out mRNA vaccines every couple months given their reactogenicity (i.e., lots of side effects). Also, continuing more of the same may reach a threshold, like we saw in the Israel healthcare study. We really need to figure out our next move. There are a few options:
Get intranasal vaccines to work (the Iwasaki lab at Yale is working day and night on this);
Roll out a different mRNA formula, like an Alpha or Omicron specific vaccine;
Start purposefully integrating a different biotechnology vaccine into the series, like an adjuvanted subunit vaccine; or,
Push for more innovative second generation vaccines, like a pancoronavirus vaccine.
Whatever the solution, we need government support. With decreased funding it’s clear that we don’t even have enough funds to purchase a fourth dose (regular formula) for everyone. Also, research, development, and innovation will soon fade without financial support. This happened with vaccine innovation after the SARS epidemic. We cannot repeat history and let this happen with SARS-CoV-2, too.
Love, YLE
P.S. I know J&J people are feeling left behind. Data continues to show that a J&J + 1 booster is working as well as the mRNA three dose series. We don’t know why, but there are many hypotheses. For example, the adenovirus biotechnology may lead to greater durability and “affinity maturation”—more sophisticated antibodies over time. Or, this may be a biproduct of mixing vaccine biotechnologies. In short, you do not need a third dose right now. We’re all keeping an eye on the literature.
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, professor, researcher, wife, and mom of two little girls. During the day she has a research lab and teaches graduate-level courses, but at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here:




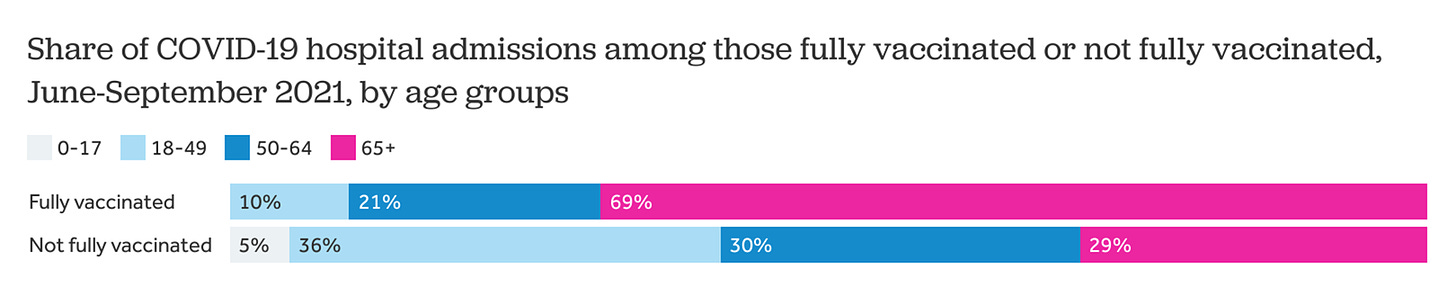
@Katelyn, a couple of thoughts and a couple requests.
Requests first: Do you have any citations I can snag on immune coverage for BA.2 derived from natural infection from BA.1? I've read several studies that I interpret in one manner but a JHU Public Health On Call podcast and their expert in that interview interpreted things differently. I'm also interested in any of the other Israeli studies on 4th-dose effect, as I recently saw one where they appeared to determine that a 2nd boost of Pfizer provided little benefit in young healthcare workers.
And, thoughts.
- Are we solely worried about circulating neutralizing antibodies, or have we settled on this because IgG is easier for the public to understand? What happened to B- and T-cell immunity and training the cellular immune system as a longer-term solution to the problem? I submit we can't keep up the cycle of repeat vaccination to maintain circulating immunity, especially now that we're seeing pharmacologic treatments that can be used without hospitalization in symptomatic individuals successfully
- The recent look at immunity afforded neonates via maternal immune response to infection as well as vaccination was interesting, especially a side note that most of the antibody response across the placenta was IgA vs IgG. I'm looking forward to the IgA -inducing vaccines.
- I continue convinced that our initial strategy of 2nd doses within 30 days of first doses of the initial dose for mRNA and likely adjuvant-vector vaccines was a shotgun approach to rapidly running up circulating IgG but failed to provide adequate time to "train" the cellular system, and failed to prime the humeral system for longer-term of circulating antibodies specific to the vaccine. Perhaps this is 20/20 hindsight but I wish we could have used a longer interval. I am now suspicious that our rapid waning of boosting doses was due that initial dosing regimen, and that we'd have seen something different if we'd been able to do second mRNA doses at 60 or 90 day intervals. Of course, it's been a long time since my immunology course work... but I'm trying to catch up.
- The issue with trying to come up with responsive variant-specific vaccines is the time it takes to ramp up production after you have a sequence. And anticipatory mRNA entries are likely to be wrong, driving up costs. In other words, we can create a new vaccine responsive to a new variant after A) the variant is identified, B) the variant is determined to be significant ("of concern" or "of high concern"), C) the variant is sequenced and the sequence distributed. Because we generally look at the Spike protein, we know where to go for changes but evaluating which of the myriad changes are significant is tedious. Almost easier to create a new mRNA responsive vaccine to the new S1/RBD elements than to try to determine which ones are at issue for vaccine evasion, transmission improvement or invoking serious disease, but inefficient in that a shotgun approach isn't enduring.
Thanks, as usual, for the update and assembling so much information in one spot.
Any idea why Israel all-cause mortality has been so high in 2022 so far? This is not a backhanded diss at the 4th shot - I am curious if you are aware of anything causing their deaths to be so highly elevated (I'm not seeing this trend in other countries).
Their excess deaths are incredibly high given the data we have from mortality.org (it lags quite a bit, so only have complete view of January and most of February, but expect weeks 6-8 to continue to rise as back-dated data comes in). They typically average 970 deaths/week with previous peaks as high as 1100-1200... yet now seeing upwards of 1300-1500 deaths/week.
Screenshot of total deaths per week: https://imgur.com/a/XVeWwgf