AstraZeneca (AZ) released a snippet of their data from the U.S. clinical trial. This press release is standard, as it typically sets in motion the scheduling for an FDA emergency authorization meeting. Some have been skeptical of the AZ trial, so this announcement was highly anticipated.
There were five important pieces of information (that weren’t previously know)…
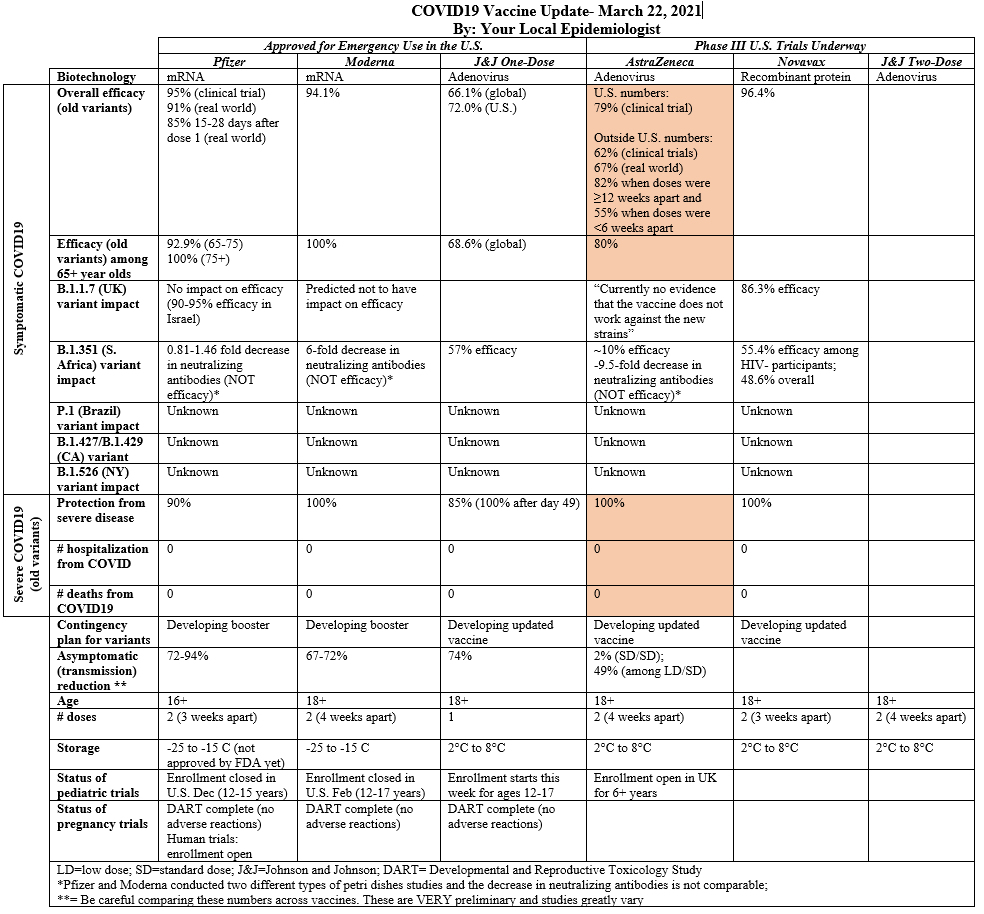
141 people got COVID19, which resulted in a high efficacy rate (79%). I assume all 141 were infected by the old variant, but this wasn’t clear. High efficacy wasn’t a surprise, but it was interestingly higher than the U.K. clinical trial (62%).
The U.S. trial stuck to the standard dose regimen (SD/SD), instead of purposefully testing the low dose/standard dose (LD/SD) regimen that accidently occurred in the UK (which needs replicating).
This is the first time we’ve seen meaningful AZ efficacy data for those aged 65+. In the UK trial, there were only 2 (out of 660 65+ participants) that got COVID19. Two people just isn’t enough to ensure causality (rather than random error), so it caused quite the debate on whether this vaccine should be recommended for those 65+. While this press release didn’t provide the raw numbers, they did make it a point to say: “it’s exciting to see similar efficacy results in people over 65 for the first time”.
The race/ethnicity distribution was a little disappointing. 79% of participants were White, 8% Black, 4% Native American, and 4% Asian. In other words, this study population wasn’t reflective of the U.S. general population. I can’t stress enough how important it is to get a representative sample in clinical trials. We need to ensure that the vaccine works in optimal conditions across genetics, cultures, environmental exposures, etc. before rolling out to the “real world”. Suboptimal representation is a chronic problem clinical trials though. This isn’t, necessarily, a AZ initiated mistake.
AZ paid close attention to blood clotting events "along with the assistance of an independent neurologist” (given recent events in Germany). They found no such events among 21,583 participants who received at least one dose of the vaccine in the U.S.
I updated our Table, with some of this new information (in peach).
That’s it for now. The FDA meeting has yet to be set, but once it is we will get to see the granular data 2 days before the meeting. I will, of course, provide the cliff notes.
Love, YLE



Are there studies showing the difference in efficacy between different vaccines for different races? I read an article about at least one vaccine having lower efficacy for Asians than for white people, but I can't find the study anymore. I ask because I'm trying to determine which vaccine I should get.
Didn’t the LD/SD show a greater efficacy before they recognized their dosing issue?
Just wondering . . . .