A critical meeting took place yesterday at the FDA. The VRBPAC (external scientific committee to the FDA) discussed one central question: What is our vaccination plan for fall? There were a number of presentations from external agencies (CDC, WHO, disease modelers) and vaccine manufacturers. Here are the meeting presentations and recording. Ultimately, the committee voted to recommend an Omicron-specific booster for fall.
Here are your Cliff notes.
The problem
We’ve been using the same vaccine formula throughout the pandemic—one created in early 2020 to fight against the original Wuhan variant. This may be okay for the future, or it may not. Coronaviruses thrive in winter, vaccine protection is waning, and the virus is changing very quickly. We don’t have a crystal ball, but all signs are pointing to a fall resurgence.
If we do need a fall booster, the FDA needs to decide ASAP to get the vaccine in arms by October, as it takes three months to manufacture and distribute. We basically have four options:
No boosters at all
Boosters with the original formula
Boosters with the BA.1/2 formula (with or without the original formula included)
Boosters with the BA.4/5 formula without testing among humans
Resurgence this fall
Waning protection. The first presentation yesterday was from the CDC and highlighted what we know about vaccine effectiveness in the U.S. Our (piecemeal) data is showing that vaccines work against severe disease, but steady erosion is occurring. And this is happening more quickly as Omicron mutates. We don’t have data on BA.4/5 yet. The CDC slide below shows effectiveness against hospitalization is 90-93% immediately after the third dose, but drops to about 73-79% 180 days later among older age groups. This isn’t a very large drop in effectiveness, but the pattern is notable. And with a highly transmissible variant, this can turn into a lot of people (as we’ve seen before).
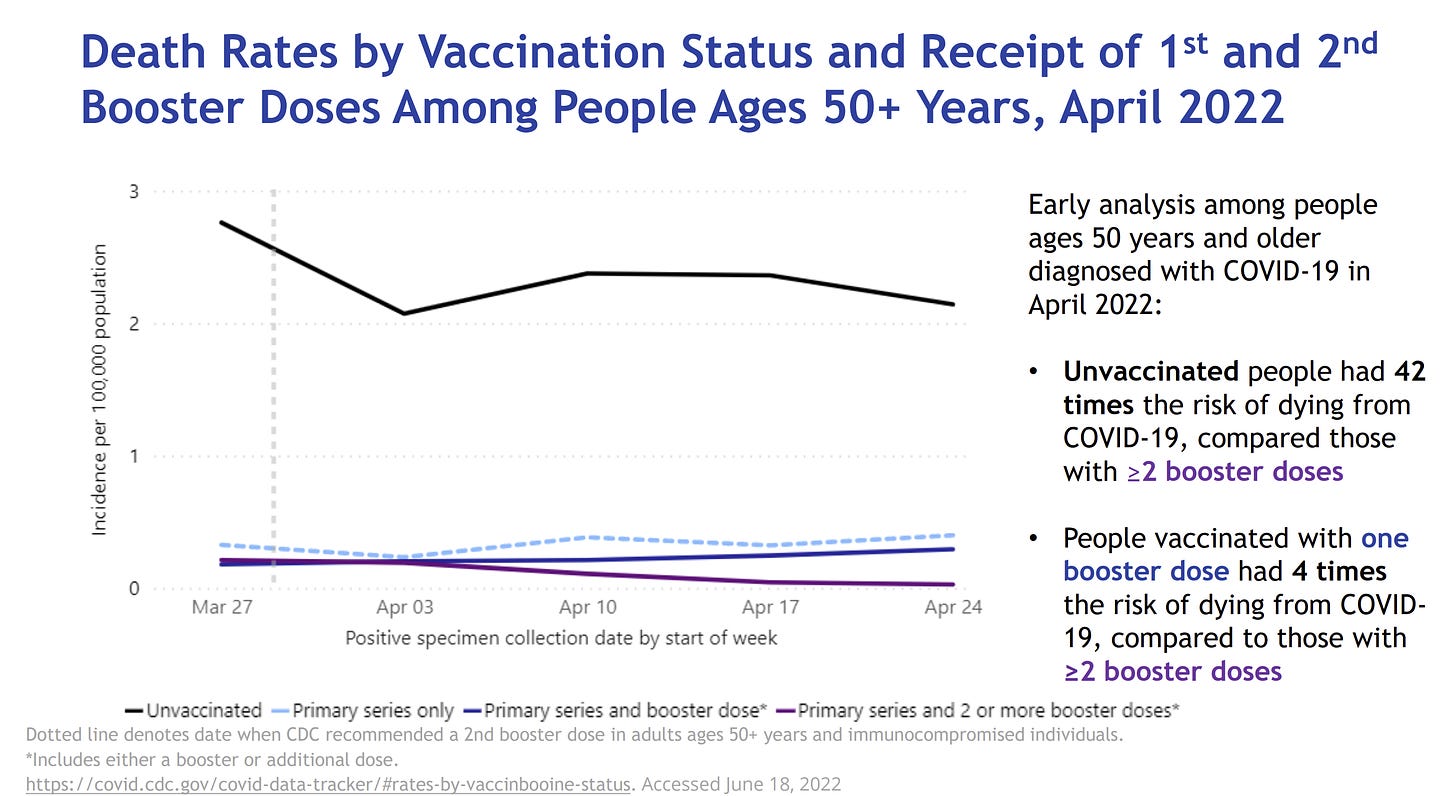
The CDC also shared that the second booster is already making meaningful impact on death among those aged 50+. People vaccinated with one booster dose had 4 times the risk of dying compared to people with 2 booster doses. (Unvaccinated people had 42 times the risk of dying from COVID19 compared to those with 2 boosters). This is consistent with data coming out of Israel.
Disease modeling. We have 20 disease modeling groups across the U.S. that are estimating different fall scenarios. Dr. Justin Lessler, a professor of epidemiology, presented the latest data, which included optimistic and pessimistic scenarios for hospitalizations based on immunity waning and the possibly of another Omicron-like event (new variant with 30% immune escape). These models do not take into account future vaccine decisions. They found:
As of now, we are trending more towards the pessimistic scenario for waning (scenario C at bottom, left below). The faster the waning, the more hospitalizations.
If we see a significant new variant, our future will look more like scenario D below. (No one really knows the probability of this happening. Some have estimated a ~30% chance.)
Regardless the model, we should stay under 100,000 hospitalizations per week, with the most likely scenario of 15,000-32,000 hospitalizations per week. Under the pessimistic scenario, we should expect 211,000 deaths between March 2022-March 2023.
Manufacturers’ answer and surprises
Vaccine manufacturers’ answer for this fall is to roll out a new booster, though their approaches slightly differ. This was their time to convince the VRBPAC committee. From previous press releases, we know that Pfizer and Moderna tested monovalent vaccines (only Omicron formula) and bivalent vaccines (Omicron + original variant formula) and that they worked well. However, the vaccine manufacturers sprinkled in a few surprises during their presentations yesterday:
Clinical trials originally tested the effectiveness of a BA.1 booster formula against the BA.1/2 virus and it worked great. But since then, a new Omicron variant has come on scene (BA.4/5). Pfizer and Moderna presented new data showing that the BA.1 booster formula is also effective against BA.4/5, but the impact was less. This was regardless of age or previous infection. (This is what we expected, given that we are seeing Omicron mutate more to escape neutralizing antibodies, but overall good news.)
Interestingly, Pfizer and Moderna came to different conclusions about needing a bivalent or monovalent vaccine. Moderna found that their bivalent vaccine was imperative for durability. The slide below shows waning was more dramatic for the monvalent Beta formula vaccine compared to the bivalent. Pfizer found the opposite: The monovalent vaccine was more effective than the bivalent. (The FDA can’t just let the manufacturers pick their favorite approach, as implementation for the public would be a nightmare in fall. The manufacturers need direction.)
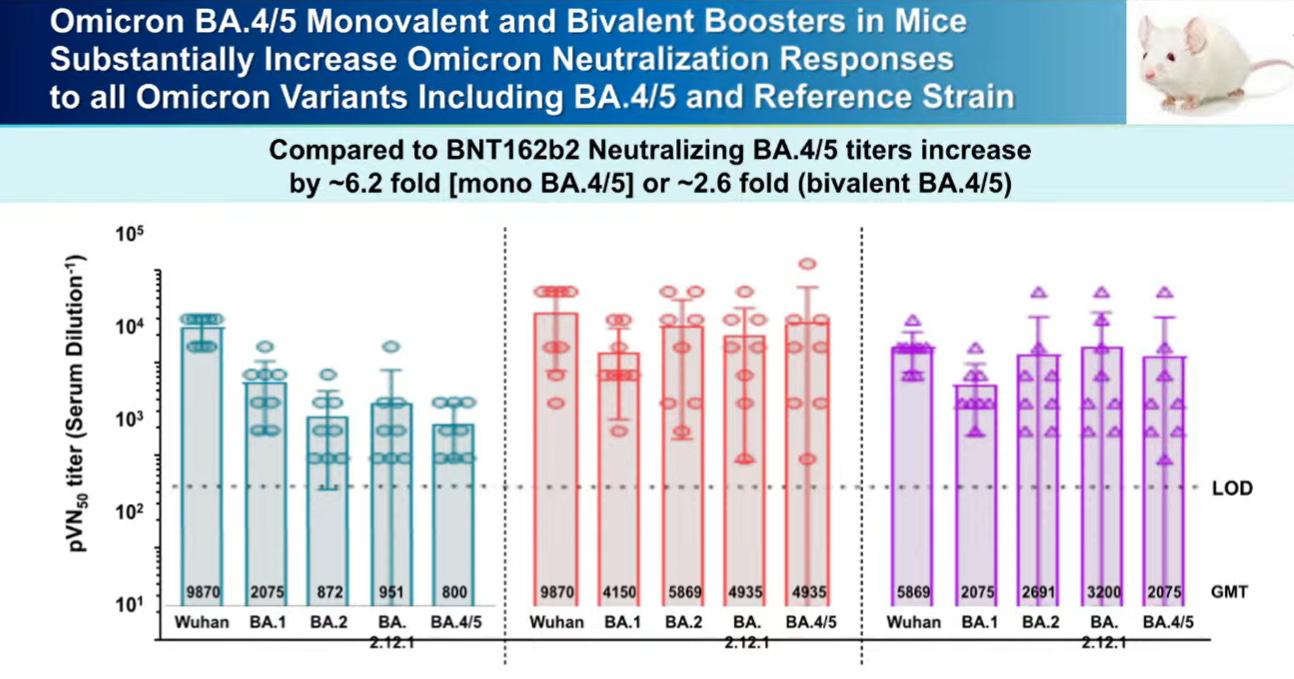
Pfizer surprised everyone and presented fresh off the press data. They already started testing a BA.4/5 vaccine formula among mice. Results below show that this vaccine worked very well against all Omicron variants. This hasn’t been tested among humans yet.
Different approach from WHO
The WHO TAG team (Technical Advisory Group on Covid-19 Vaccine Composition) was then invited to present. With the flu, the WHO makes an annual recommendation before the flu season arrives. This attempts to get everyone on the same page. And everyone has always agreed on the approach: attempt to match a vaccine formula to circulating variants. However, during their presentation yesterday, it was clear the WHO is not trying to force a flu framework for SARS-CoV-2. This virus is mutating too fast. Their goal of a fall booster is to broaden protection. This is different from FDA’s approach to chase SARS-CoV-2 variants—get a vaccine to match circulating viruses. Because of this, the WHO is recommending a fall monovalent or bivalent booster with the BA.1 formula instead of using a BA.4/5 formula. (I must say, I agree with WHO’s approach. We aren’t going to “win” a rat race with this virus.)
Discussion
So what do we do for fall? After the presentations, a ton of questions and comments ensued among the committee. This simply reflected the complexity and difficulty of the situation. The discussion had a few themes:
No clinical data. Some voiced concern that we only have data on neutralizing antibodies. We don’t know, for example, if a 1.75x improvement provides clinical improvement. (Note these uncertainties were similarly voiced for booster 1 and booster 2. But we see that the boosters continue to have a meaningful impact on preventing death. Mounting scientific evidence shows neutralizing antibody levels predict severe disease. We can’t wait for ideal data; we need to make decisions based on imperfect data.)
Broader data. There was also discussion around how we don’t have data on T-cell or B-cell response. The FDA continues to say this is too difficult to measure and standardize, and even more difficult to map to effectiveness in the real world. We really need FDA’s leadership to change this. We need a more comprehensive picture to make better decisions moving forward.
Making the jump. Some members said they weren’t comfortable recommending the BA.4/5 formula without clinical data. This is done each year with the flu vaccine (we don’t need clinical trials), but members said this is still a new vaccine and we need the data. It seemed, though, that the majority were in favor of a BA.4 or BA.5 formula vaccine. (I agree. These vaccines are so similar to previous ones that we can skip clinical trials. Obviously we need a definition of when a vaccine is not “new” anymore.)
Kids. There was some discussion on how to get everyone on the same page with boosters. The age de-escalation phases were necessary for the first vaccine series, but kids can’t continue to be 1.5 years behind. There was a mix of opinions whether we can confidently use adult data for rolling out pediatric vaccines. The vast majority agreed that the vaccine manufacturers need to collect pediatric data more quickly. (We need to start enrolling kids in trials with adults from here on out.)
Age. Some voiced support for an Omicron vaccine only for older age groups. Who gets a vaccine will be determined down the line (probably by ACIP).
After discussion, they voted on the question: Does the committee recommend inclusion of a SARS-CoV-2 Omicron component for COVID-19 booster vaccines in the United States?
Yes: 19
No: 2 (Drs. Offit and Bernstein)
Abstain: 0
The FDA will make the final decision. Looks like we are getting an Omicron vaccine in the fall. It will likely be a bivalent vaccine and probably with BA.4/5 formula. Who will be eligible is yet to be determined. Could we wait for a booster? Maybe. Should we wait is a different question. I think this is the right call.
Love, YLE
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank, and at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here:








Not quite clear to me if you favor a BA.1 (per WHO) or a BA.4/5. I am probably reading it incorrectly.
It is one thing to roll out a booster for the public. But if we learned anything in the last two years, it is that it is another, more difficult task, to get the public to accept the shot. The WHO and the FDA must take shot reluctance into the equation and minimize the traction any counter propaganda the anti-science community comes up with. This project is part very high level bioscience and part lo-tech marketing.