The Pfizer vaccine is now authorized, by the FDA, for emergency use among people aged 12+. In other words, you could go right now and get a vaccine in your 12 year old’s arm (dependent on your state and physician, I’ll get to that in a little).
However, the CDC advisory board (called ACIP) has yet to meet (they are meeting Wednesday; here is the agenda).
So, what’s going on?
In normal times…
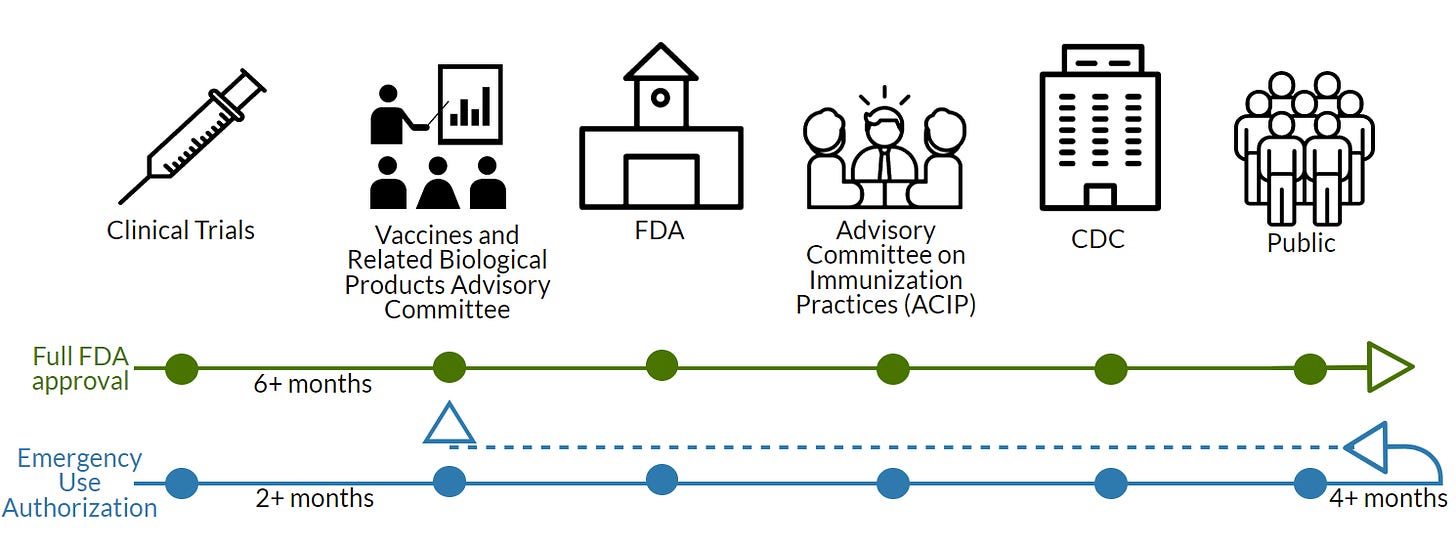
A vaccine sponsor (like Pfizer) would collect at least 6 months of follow-up data from Phase III clinical trials and submit an extensive application to the FDA. Then, the FDA would have 10 months to review, approve, and license. During this time it’s federally mandated that an external review board for the FDA (called VRBPAC) has a meeting. This is where we (the public) get to see the clinical trial data for the first time (hundreds and hundreds of pages). VRBPAC provides a formal recommendation to the FDA.
Then an external committee for the CDC (called ACIP) offers a second recommendation. The CDC Director has to ratify the ACIP decision. The CDC typically needs to approve for three reasons:
Insurance to cover the vaccine
Government funds to be adjudicated to pay for the vaccine for uninsured kids (called the Vaccines for Children program)
CDC handles the logistics for delivery of vaccine, so they have to formally approve what they are distributing.
In abnormal times…
If there’s a pandemic or global emergency, a sponsor can apply for an Emergency Use Authorization (EUA). The sponsor only needs 2 months of follow-up clinical trial data to apply. Then, the rest of the process is basically the same.
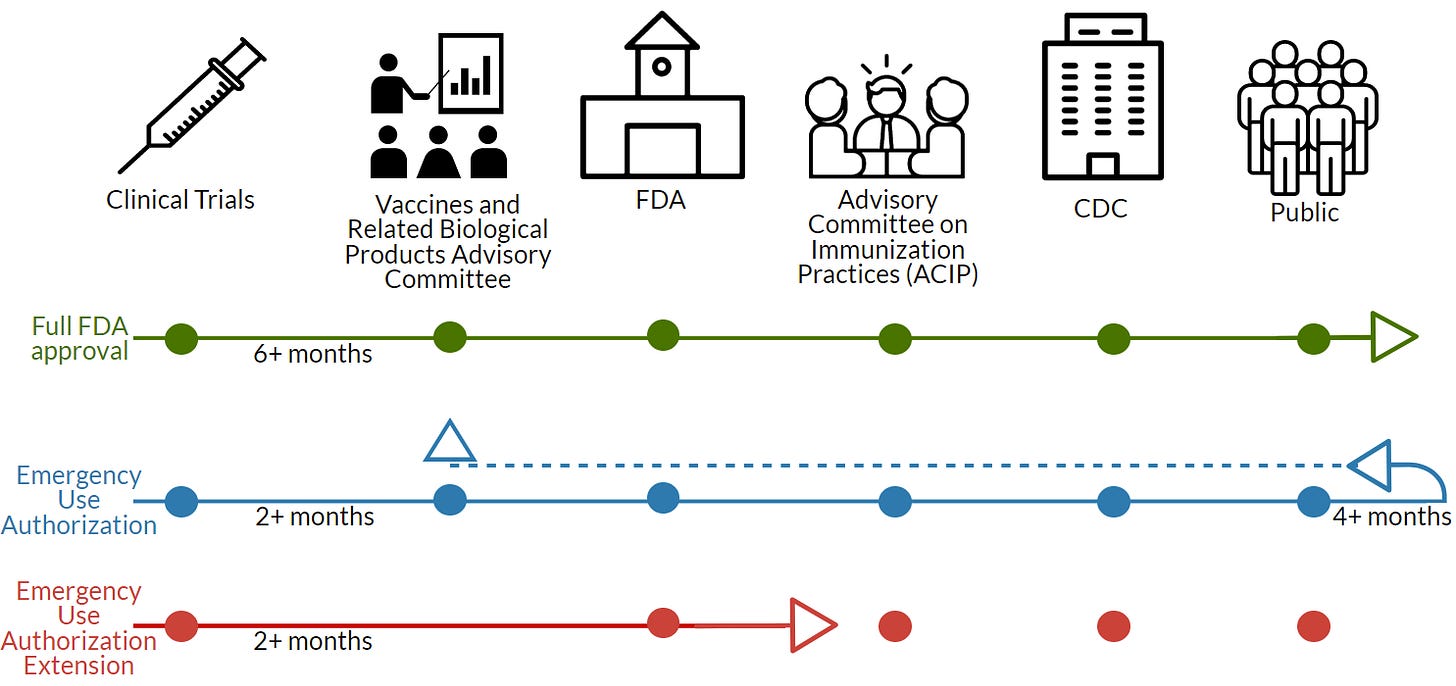
When a sponsor applies for a EUA, it’s under the assumption that the sponsor will apply for a full license once they have the follow-up data (showing vaccine longevity, continued safety) and necessary documents (like manufacturing processes). Then the FDA has 6-10 months to review. This is where the adult Pfizer vaccine is right now.
But this is abnormal, abnormal times…
The adolescent vaccine is NOT a new EUA; it’s an extension or an amendment of the adult EUA. So, the FDA doesn’t require a VRBPAC meeting. The FDA internally reviewed data and deemed it safe and effective for emergency use (which led to today’s announcement).
The ACIP meets Wednesday, which is basically a formality. Importantly, though, we (the public) will get to see some data. In some states, only pediatricians can give vaccines to kiddos (not pharmacies). Also, a LOT of physicians will still wait for the ACIP recommendation. So, this CDC meeting is still an important step.
Bottom line: The Pfizer vaccine is officially authorized for patients. We will still get a lot of new and important information on Wednesday.
I hope I didn’t make this already confusing process even more confusing.
Love, YLE





Thank you! Confusing still - but a lot less confusing than it was. It is helpful to hear that it might be worth waiting until Wednesday's info before scheduling a jab for a 12-y-o! I really appreciate your taking the time to help us non-medical people wade through all of this!!
Appreciate ALL the information you give and the hard work you put into these substacks!! Ready for Wednesday and more information! TY!!!