Today a much anticipated meeting took place at the FDA. Their external scientific committee—called VRBPAC—voted unanimously to update the COVID-19 vaccine in anticipation of the fall 2023-2024 respiratory illness season.
Here are your Cliff notes.
Why update the COVID-19 vaccines?
We could keep the same vaccine formula this fall, but several reasons we should update were presented:
SARS-CoV-2 continues to mutate quickly—about 2 times faster than the flu. It’s normal to update vaccines when the virus mutates quickly. For example, we update vaccines for flu (which changes ~annually) and we don’t update vaccines for measles (which hasn’t mutated in a meaningful way for decades).
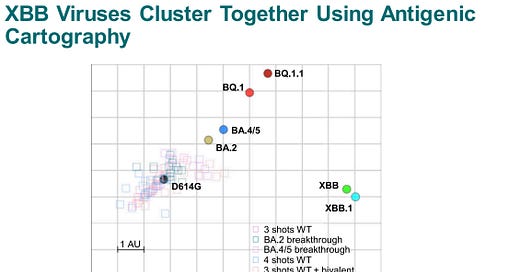
The current Omicron variant (XBB) circulating is meaningfully different than other Omicron variants. The map below shows the differences, with XBB pretty distant. This suggests an updated vaccine with XBB would help our immune systems recognize the change.

COVID-19 vaccines are waning in protection against hospitalization (62% effectiveness → 24%) and ICU admission, albeit with a smaller decline (69% → 52%). This is happening faster when exposed to XBB virus compared to other Omicron variants.
But it’s really important to recognize that these numbers are relative. Even though vaccine effectiveness is waning, the hospitals aren’t filling up. This is because vaccine effectiveness now represents the incremental benefit above and beyond the baseline protection in the general population. This is different than when we first introduced vaccines and the general population had a very low immunity wall.
Helps with other layers of protection. Antibodies aren’t the whole story; getting data on our other layers of defense, like B-cells (antibody factories) and T-cells (slower to respond but important for severe disease), has been difficult.
I was happy to see data on these presented today:
B-cell data showed that our antibody factories are able to adapt and pump out updated antibodies. In other words, there is imprinting from initial exposure (as expected) but our system is still adaptable. This is good news because it means that updated vaccine formulas expand our protection. It’s not all for nothing.
T-cell data also showed clear 2-fold increases after an updated booster. This was the case regardless of prior infection.
Bivalent to monovalent… again?
The original vaccine was monovalent (vaccine formula targeted one variant—Wuhan). Then, in 2022, the vaccine formula was updated to bivalent (targeted two variants—Wuhan and Omicron BA.4/5). Now, the FDA wants to go back to monovalent (targeting only Omicron XBB).
Why go back to just one strain? A few reasons:
WHO is not seeing any evidence that the earliest variant is still circulating. So we can drop that from the formula. In fact, including it could hurt as we keep teaching our immune system to recognize the old version of the virus rather than the new one.
There really isn’t anything else popping up except XBB variants. It’s smart to focus on this.
Novavax found that a monovalent vaccine may be more advantageous to mice’s immune systems than a bivalent vaccine. Moderna found the same thing.
There is the possibility of a variant popping out of nowhere, which would favor putting another variant into the vaccine formula. But what variant? Predicting this is close to impossible.
Other interesting snippets
Novavax will be an option this fall. I know people will be happy about this.
Nextgen vaccines are at least 2 years away, if we are lucky.
Everyone’s curious to see how this year plays out. The rest of the year will be very telling as to whether COVID-19 has settled into a seasonal, predictable pattern.
Comparing COVID-19 to the flu is helpful, but the viruses, and thus processes, are different. This needs to be communicated better. Perhaps worth a YLE post.
Don’t let anyone tell you there are no human data on these vaccines. All vaccine manufacturers presented preliminary data on humans and animals. What we do not have yet is the safety of these vaccines when combined with RSV and flu vaccines.
There seems to finally be global alignment. This was not the case last year when the WHO recommended BA.1 and the U.S. chose BA.4/5 vaccine formulas. This year, the U.S., E.U., and WHO seem to agree on XBB, which is a welcome development.
Communication. Someone needs to help the FDA communicate all this. As one member said, we need to ensure we are “sending the right messages and setting the right expectations” for the public, including risk communications. (Yes, yes, yes.)
Vote
All VRBPAC members (21) voted in favor of updating the vaccine formula to XBB for fall.
So, what’s next?
Pfizer, Moderna, and Novavax will start manufacturing millions of vaccines. Once they’re ready, the FDA will approve the updated vaccine. Then ACIP will determine who should get the vaccines. Expect this to happen in late summer or early fall.
Bottom line
Expect an updated COVID-19 vaccine formula this fall that targets the XBB Omicron variant. One massive question remains, though: Who will be eligible? We will know in a few months. Stay tuned.
Love, YLE
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, data scientist, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank and is a senior scientific consultant to a number of organizations, including the CDC. At night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well-equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support this effort, subscribe below:





Fantastic summary! And in regard to your statement that someone should help the FDA explain these findings to the public, that someone is definitely YOU! Maybe they would also want a simple 5 word feel-good statement for those in the public that don't have the time or inclination to digest a more complex answer, but for those of us who realize that Covid killed > one million Americans and want to do something about it, you are a lifesaver!
Why will there need to be any discussion about WHO will get the vaccines? We are all at risk from COVID. This shouldn’t even be a conversation at Year 4 of this ongoing pandemic.
As a parent of young kids, I will be LIVID if the so-called public health apparatus decides to leave out our kids AGAIN.