Today the Advisory Committee on Immunization Practices (ACIP) had an emergency meeting regarding the Johnson and Johnson (JJ) vaccine and blood clotting events. In short, three major questions needed to be answered:
What happened?
Is this rate higher than the background rate?
Do we need to change our recommendations?
All in all, this process solidified by confidence in the vaccine process in the U.S. The amount of transparency, timeliness, and checks and balances has been refreshing.
Here are your meeting cliff notes…
Who was present?
Voting members are importantly external to the CDC, FDA, and JJ: mainly professors, epidemiologists, physicians (genecology, pediatrics, infectious diseases), public health professions at health departments, infectious disease specialists, and health policy specialists. One voting member reported a conflict of interest and was not allowed in the final vote (nor was able to deliberate). Also present was the CDC, FDA, JJ, Health Resources Injury Compensation program, NIH, and many others including international societies.
What happened?
While there are many types of blood clotting events, we’re interested in one specific event: CVST with thrombocytopenia (low blood platelet count). Basically, this event prevents blood from draining out of the brain and causes a brain bleed. And, because of low platelet counts, patients have internal bleeding that can be fatal.
Out of the JJ research studies (343,000 people) and the “real world” (7,200,000 people), there have been 9 cases of CVST
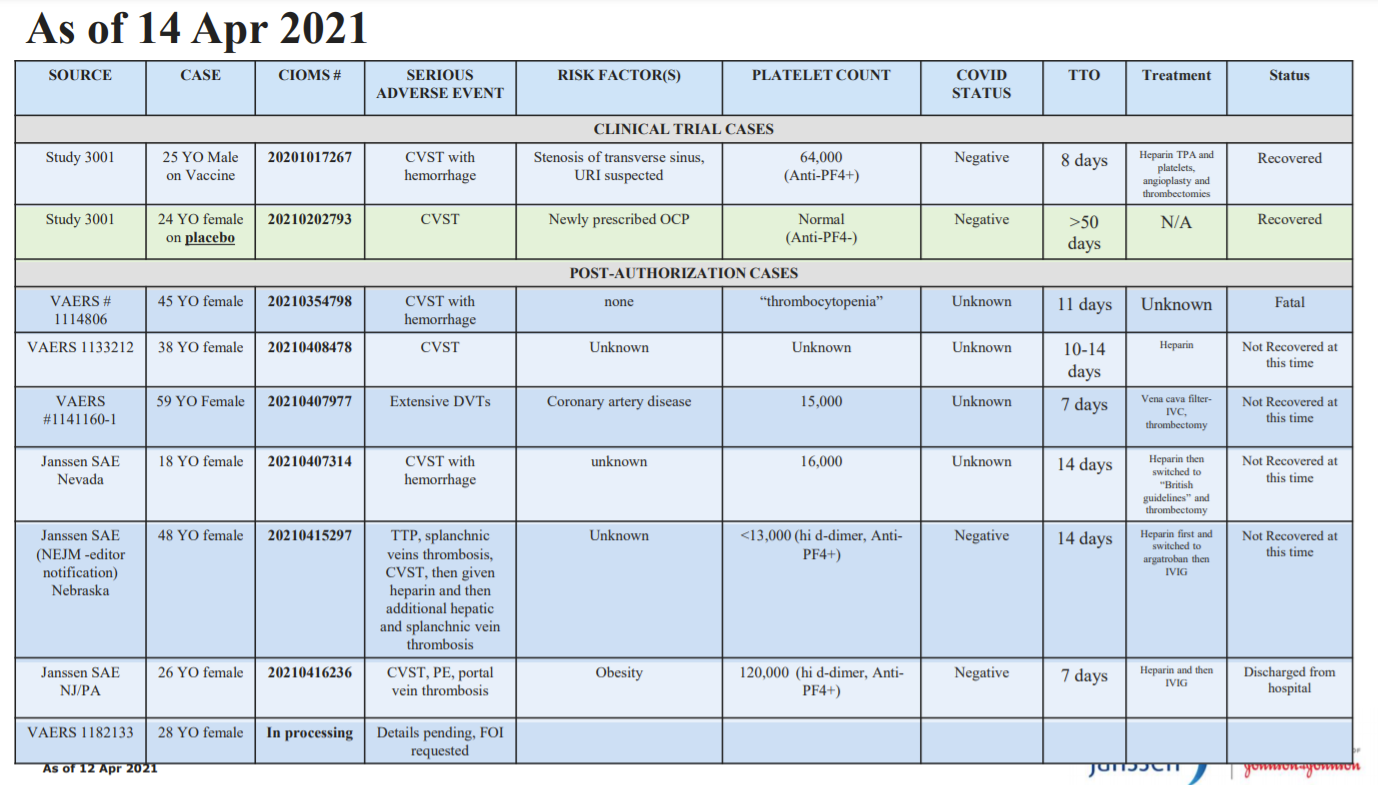
Random notes from the 6 post-clinical trial cases:
We don’t know whether heparin caused the fatal outcome
Out of the 6 post clinical trial events:
Only one person was on birth control
None had pre-existing coagulation disorders
Interestingly in JJ’s other programs that use adenovirus (like the Ebola vaccine), there are no CVST events.
Is this rate higher than the background rate?
This will help us understand whether the CVST events after vaccination is higher than in the general population.
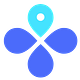
In the general population, CVST is rare. In old studies, background rate is estimated between 2-5 per million. More recent numbers are upwards of 15 per million.
It is more common among younger females.
The problem with these numbers, though, is that these numbers are for CVST cases overall. NOT CVST low platelet count (or thrombocytopenia). So, 2-15 per million background rate is too high, but the epi team is working on getting a better estimate.
Let’s just say 2-15 per million is the background rate (although we know this is too high). Given this, we are seeing between a 3.8-15.2 fold higher rate of CVST among 20-50 women years after vaccination compared to women aged 20-50 in the general population. This likely indicates that the JJ vaccine does cause CVST with thrombocytopenia.
Do we need to change our recommendations?
There are two questions that the ACIP needed to vote on.
First, does the ACIP have enough information to make an interim recommendation?
If yes, then what recommendation does ACIP feel is appropriate today given available information? In that case, we have four options, all of which have pros and cons:
Extend pause
Stop distribution for everyone
Continue distribution only among a certain population (this is what a lot of European countries chose for AstraZeneca)
Continue distribution for everyone
An interesting discussion ensued. Many voting members were very verbal in stating that we don’t have enough information about the risk/benefit balance and we need more information.
We are fortunate because we have other vaccines available. Because of this, we can be cautious and, first and foremost, do no harm
Usual treatment (heparin) is harmful, so we can’t just say we can treat it if more cases come up
We don’t know about age yet (it might be underreported or not flagged for those over 50+)
We don’t know what these rates are among those that already had COVID19 disease.
Unfortunately, though, this vaccine is best for the most vulnerable. The most vulnerable will continue to be at risk is this is paused. This pause would continue to introduce inequity.
VOTE. JJ will remain paused until we can get a formal risk/benefit analysis. Hopefully, ACIP will meet within 10 days to make a decision.
Love, YLE




Thank you so much for all your updates. Especially this one. I've been deeply curious about what the background rate was for CVST, and prior to reading your wonderful details hadn't recognized CVST and CVST with low platelets was a significant difference and why.