Today the external review board for the FDA (called the Vaccines and Related Biological Products Advisory Committee) met to answer two important questions:
Is a 3rd Pfizer dose safe?
Is a 3rd Pfizer dose effective?
The meeting consisted of a number of presentations, including from the CDC, UK, Israel, Pfizer, and FDA. Then this went into a Q&A, discussion, and vote.
Is it safe?
Pfizer shared their 3rd shot clinical trial data, which showed side effects were about on par with the result from the 2nd dose. In short, about 64% of people that get the 3rd dose should expect fatigue, 48% should expect a headache, and 40% should expect chills and muscle pain.
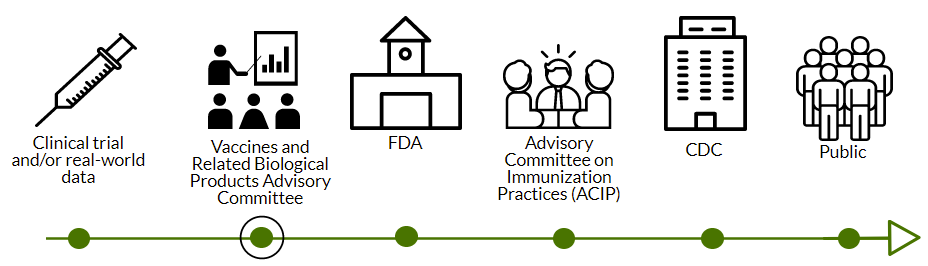
Israel presented some of their “real-world” data on severe adverse events after a 3rd dose. Overall, 19 severe adverse events occurred in Israel following 2,870,272 people who received the 3rd dose. Two events (allergic reactions) were caused by the vaccine; Five other events are still being investigated.
Israel reported 1 myocarditis case out of 1.1M 30+ year olds who received a 3rd dose. This was a male patient in his early 30s who had chest pain and fever 3 days after vaccination. No other cases have been observed thus far.
Is it effective?
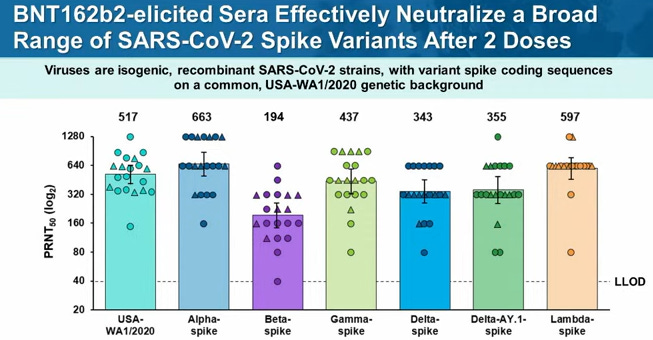
From the Pfizer clinical trials, the 3rd shot clearly increased neutralizing antibodies.
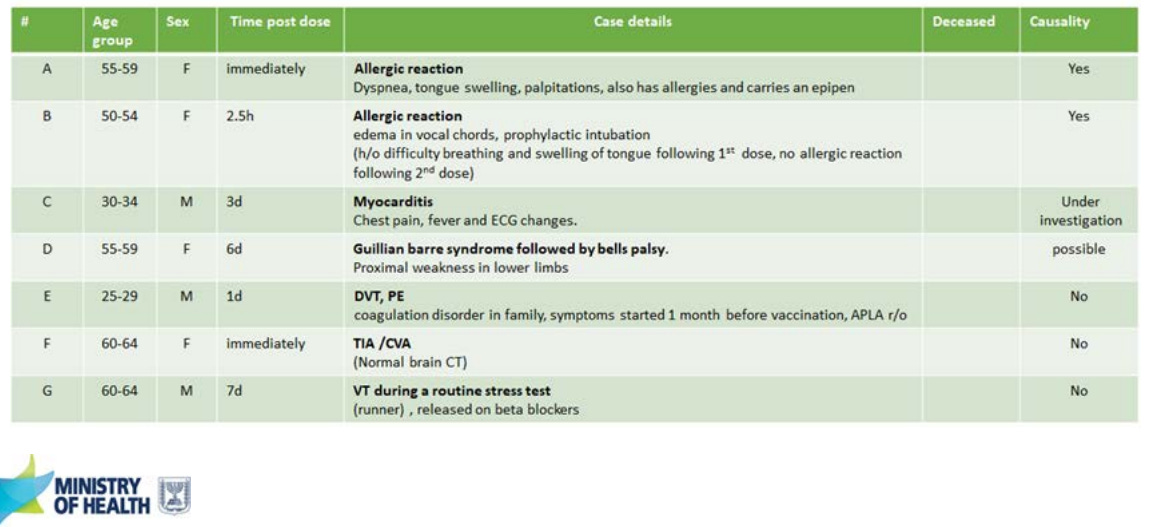
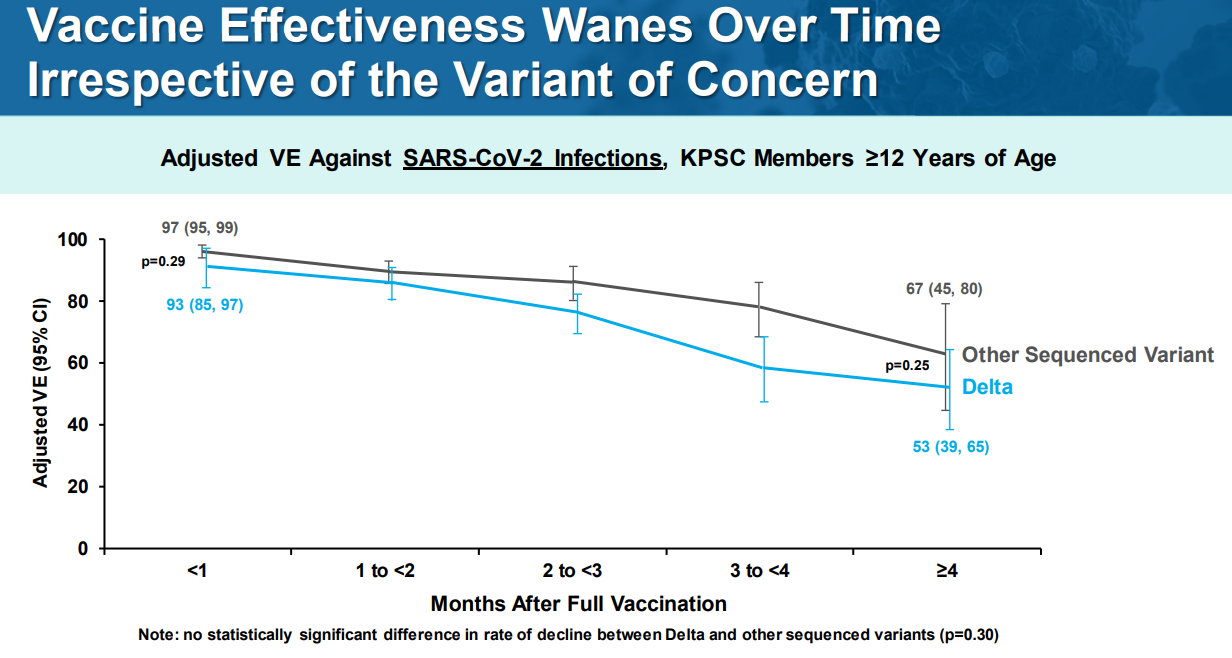
We got a fantastic look into the “real world” effectiveness of a 3rd dose from the Ministry of Health in Israel. In early August, severe cases started to dramatically increase 6 months after the majority of the population got a 2nd dose. Israel ran projections showing that without a booster, hospitalizations and deaths among vaccinated would continue to rise. After the 3rd dose, the rate of severe disease among vaccinated started to decrease.
It was very clear from their presentation that Israel’s policy decision was also based on two other goals:
Reducing transmission (getting R(0) below 1); and,
Preventing long COVID19.
Q&A
After presentations, voting members were able to ask the CDC, FDA, Pfizer, UK, and Israel representatives questions. This is where we got a lot of important answers. Here are a few:
Why is there so little reporting on cellular immune response instead of just neutralization antibodies? What evidence does Pfizer have? And what does cellular immune response look like for the average person? Pfizer responded by saying it had great data on memory B-cell and T-cell response after immunization. B/T-cell response is working great among the general population. B-/T-cell immunity isn’t good at protecting against infection and spread. We need neutralizing antibodies to do this.
Why not make the 3rd dose for the Delta variant specifically? Pfizer said Delta is not escaping immunity like Beta is/was. There is scientific evidence that protection is not just waning for Delta, it’s waning for other variants too. So, this isn’t mainly a Delta problem; it’s more of a timing problem.
Why don’t we know the specific antibody titer needed for protection? Israel is studying this right but, but won’t have results for another month. Pfizer has looked at this and were unable to come up with antibody threshold. They said this is a much more complicated story than neutralizing antibodies.
Discussion
Then a discussion among only voting members ensued. Frustrations, really centered around data, were aired:
Members discussed Pfizer’s surprising answer that they, in fact, had B/T-cell data and didn’t share it in their presentation. If B/T-cells are mounted, then the general population would have protection for a long time. So, this gets even more confusing… Why is Israel’s vaccines not working against severe disease? We desperately need to see Pfizer’s data on B/T-cell protection.
Voting members voiced frustration about lack of safety data. Particularly, the risk-benefit ratio for myocarditis. The Pfizer clinical trial of 3rd shots was very small sample (~300 people). So no reassurance was provided for myocarditis. But, unfortunately, this is only really able to be answered post-licensure approval. Vaccine-induced myocarditis is so rare that we need millions of people vaccinated to see the “true” effect. An in-depth benefit-risk analysis could provide insight into whether the benefit of vaccinating younger with a 3rd dose is better than the risk of potential myocarditis from a 3rd dose. This was missing from the presentations today.
While Israel started to see increase in severe cases, we haven’t seen that in the United States. But then others questioned…do we want to wait to see if this happens in the United States?
A third dose may benefit transmission. But we haven’t seen this data. If we had this data, it would really change the game that we could (or could not) impact R(0). If we want to stop the pandemic, will vaccinating the vaccinated again really make a meaningful impact? To make the biggest impact we need to vaccinate the unvaccinated. Some members stated that this transmission could help with the current pediatric cases surge while we wait for the pediatric vaccine.
Vote
So, the committee needed to vote: “Do the safety and effectiveness data from clinical trial support approval of a booster dose administered at least 6 months after completion of the primary series for use in individuals 16 years of age and older?”
YES: 2
NO: 16
It did not pass. Many voting members votes “No” because of the age definition in the question. They rather a booster for older adults right now. Many members admitted they think a 3rd dose is coming, but need more data.
So they came back for a second vote. They ultimately decided that the 3rd dose was necessary for a few groups:
65+
Certain medical conditions
Certain occupations (like healthcare)
So, this committee’s recommendation will go to the FDA, who will ultimately make a decision. Then this meeting will go to the CDC next week for a vote.
Have a great weekend, YLE
Here was the meeting information. Here are the presentation slides.








Thanks for updating your lede line to avoid confusion!
I watched the meeting and it seems like in a second vote they recommended a 3rd dose for 65+, at high risk for severe covid, and at high risk of occupational exposure (healthcare workers, etc). Is that accurate?