Our little company from Maryland pulled through!
Novavax is a small company based in Maryland and has never brought a vaccine to market. This company has a fascinating rollercoaster history; I highly recommend googling over coffee. Briefly, it’s a company that almost lost it all, but making a comeback during the pandemic.
A third “type” of vaccine
Novavax is using a different vaccine formula than Moderna, Pfizer, AstraZeneca, and J&J. The Novavax vaccine contains a cornavirus protein that prompts the immune system. Scientists combined this protein with an immune-boosting compound derived from the soapbark tree. They tried doing it without this adjuvant but the vaccine didn’t work as well. Anyways, this type of vaccine has a much longer track record than the newer approaches.
The exciting (and innovative) aspect of this vaccine is that Novavax found a way to make this vaccine in moth cells (rather than mammal cells). The moth cells basically become little factories that pump out coronavirus proteins. This allows Novavax to manufacture the vaccine much quicker than others, which is obviously a big plus in the time sensitive pandemic
And, clinical trial results look AMAZING
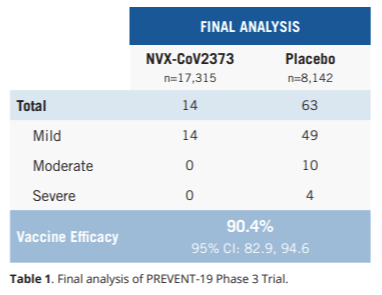
The trial, called PREVENT-19 (PRE-fusion protein subunit Vaccine Efficacy Novavax Trial COVID-19), enrolled 29,960 participants 18 years of age and older in 119 locations in the United States and Mexico. People were randomly assigned to the vaccine or the placebo. Here are the results…
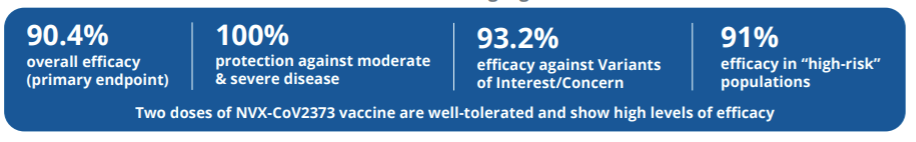
Efficacy was high = 90.4% (77 total COVID19 cases: 63 in the placebo group, 14 in the vaccine group)
Moderate or severe disease efficacy was 100% (10 moderate cases and 4 severe cases, all in the placebo group)
Among “high-risk” populations, vaccine efficacy was 91.0% (62 COVID-19 cases in the placebo group, 13 COVID-19 cases in the vaccine group). “High-risk” was defined as over age 65, under age 65 with certain comorbidities or having life circumstances with frequent COVID-19 exposure.
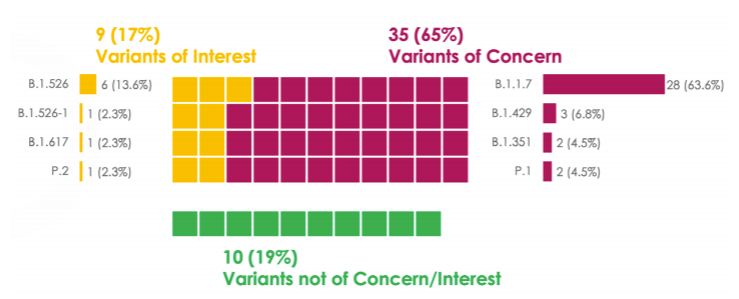
This high efficacy across all categories is incredibly impressive (even when comparing to Pfizer and Moderna) because this trial was done with circulating variants of concern (VOC). During the clinical trial, Novavax genetically sequenced 54 of 77 cases. 65% were VOC, 17% were variants of interest, and 19% were non-VOC.
Among non-VOC, efficacy was 100%
Among VOC, efficacy was 93.2% (38 of the VOC cases were in the placebo group, 6 were in the vaccine group)
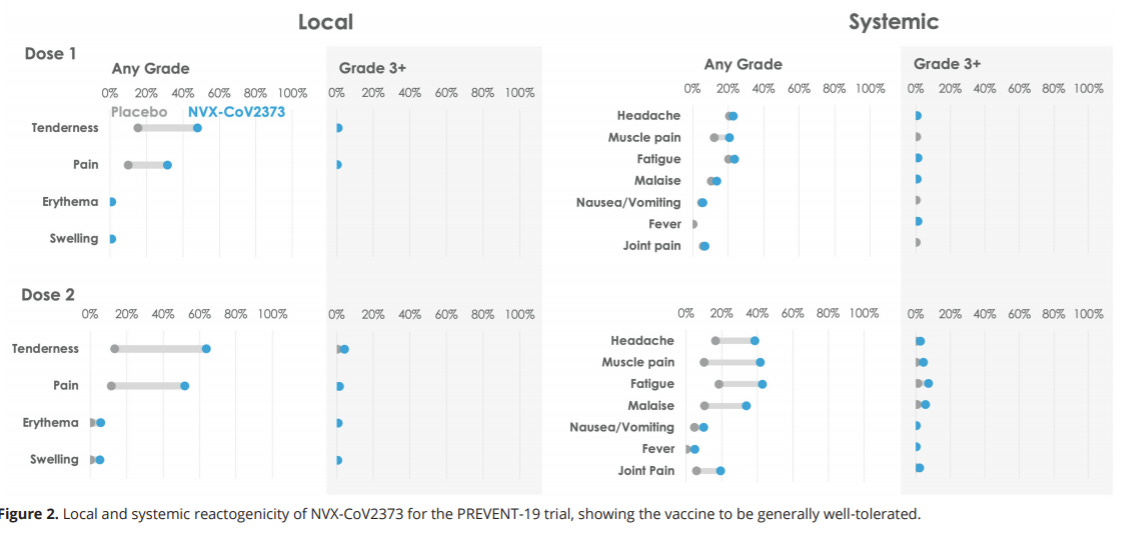
Novavax is safe
Side effects look much lower with Novavax compared to mRNA and J&J vaccines
There were a low number of serious and severe adverse events, but they occurred equally in the vaccine and placebo groups (i.e. were not due to the vaccine)
Bottom Line(s)
I cannot overstate how ecstatic we are with the efficacy of this vaccine, especially with the VOC. We may even want to consider Novavax for our booster shots in the U.S.
It sounds like Novavax will wait to apply for emergency use authorization for a few months, and even so, it’s not clear whether the FDA would authorize it. They might have to go straight for full approval. Novavax IS applying for emergency approval in other countries though. It will, no doubt, help us fight the global pandemic given only 15.4% of the world is vaccinated, given the high efficacy with VOC, and given normal temperature storage distribution implications.
Finally, who doesn’t love a story about an underdog pulling through.
Love, YLE




I am in this trial as well! I barely ran a fever at all (only 100.5) and only had super super mild body aches for a few hours. Like Andrew, I wish we could get FDA approval b/c I don't have a vaccination card yet but I know I'm fully vaccinated after going through the blind crossover. I'm super hopeful this will become the booster and I hope the pediatric trials are just as successful b/c it would be AMAZING for kiddos to not have to experience the unpleasant side effects you see in the other vaccines. #GoTeamNovavax!!!
I’m thrilled to see these results as I am a PREVENT-19 trial participant. I had absolutely no side effects and was convinced I got the placebo until crossover was done but it turns out the vaccine is just that good.
I’m sad to see the delays in FDA application because I know a few people who aren’t getting vaccines due to side effects, fear of the mRNA, etc. When I told them about my experience and that the ingredients are tree bark and moth cells they sounded more interested. I hope the world can benefit from this great option.
I also hope it gets approved in the US eventually because us trial participants can’t receive an official vaccine record for investigational products.