Pediatric Vaccines: FAQ from parents
Between the hundreds of messages and interview questions I got yesterday, I synthesized FAQ re: pediatric vaccines. Here are a few answers to your fantastic questions…
“Could the United States decide NOT to authorize the vaccine for 5-11 year olds?”
Yes, this is a possibility. I am hopeful we’ll get vaccines, but there are a few places where this process could be stopped/delayed:
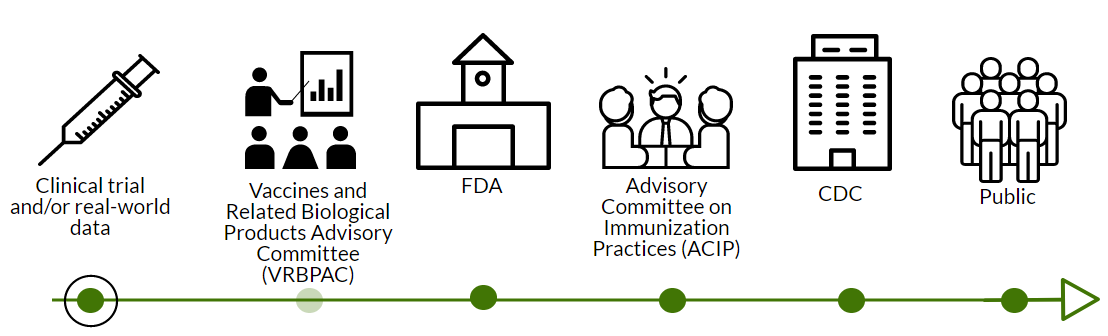
EUA application: Pfizer will submit an Emergency Use Authorization (EUA) amendment for 5-11 year olds. Originally, the FDA hinted they would not consider an EUA for kids <12 years. So, Pfizer was planning for a full biological license application (BLA). But, Delta and pandemic resurgence may have caused the FDA to change perspectives. If the FDA does not approve the EUA route, Pfizer will need to apply for full approval. This would require 6 months of safety data, which we don’t have right now. I don’t think this will happen.
FDA Review: If the EUA route is approved, the FDA will review the loads of data from Pfizer. This takes about 4-6 weeks. This process is typically much longer but the FDA has said they’re prioritizing this effort.
Then, the FDA could call VRBPAC. VRBPAC is an external scientific committee that would review the data to give their opinion about the safety and effectiveness of the vaccine for 5-11 year olds. This step was not conducted for the adolescent Pfizer vaccine; VRBPAC doesn’t have to be called for an EUA amendment, but they could. Given the VRBPAC booster meeting last Friday, this would make me nervous. It was clear from the booster discussion that VRBPAC wants clear, rigorous data to make decisions. Only two voting members recognized that we sometimes have to make decisions based on limited data. VRBPAC could decide (through a majority vote) that we don’t have enough safety data on myocarditis to allow kids 5-11 to get vaccines. The FDA makes the final decision, but rarely (if ever?) do they go against VRBPAC’s vote. There is a lot of power at play here.
CDC Review: If the FDA doesn’t call on VRBPAC or VRBPAC votes that this vaccine is safe and effective, then this goes to the ACIP (an external scientific committee for the CDC). ACIP assesses safety and effectiveness too. They also play a key role in policy (who, what, where gets the vaccine; global equity considerations; etc). ACIP is amazing at conducting rigorous risk-benefit analyses, even with limited data. So, we’ll get a fantastic look at whether risks outweigh benefits for 5-11 year olds. Then ACIP will vote on whether to recommend the vaccine to 5-11 year olds. CDC makes the final recommendation, but rarely (if ever?) goes against ACIP.
Then assuming that the FDA and CDC agree, we would get vaccines in 5-11 year old arms by Halloween.
“What did the clinical trials assess?”
In Phase I of clinical trials, Pfizer needed to find the right dosage. Kids are not tiny adults. They have very different immune systems and we wanted the biggest bang for our buck (i.e. smallest dose for the best antibody response). So, 114 volunteers aged 5-11 were given one of three options: high dosage (30 mcg; same as adults); mid-dosage (20 mcg); and a small dose (10 mcg). They were given the same dosage 21 days later. Pfizer found that the smallest dose (10 mcg) worked fantastic.
Once Pfizer had Phase I results, they could move onto Phase II/III. In this phase, 4,500 children were enrolled at more than 90 sites in the US, Finland, Poland and Spain. But, right now, Pfizer has data on 2,268 participants with at least 2 months of follow-up data. This is enough kids with enough follow-up data to submit an EUA. (This metric is predetermined by the FDA).
In Phase II/III, scientists compared 5-11 year olds’ antibodies after 10 mcg to 16-25 year olds’ antibodies after 30 mcg dose. From yesterday’s press release, Pfizer said that the 10 mcg dose mounted a strong immune response (the same immune response as adults) with no serious adverse events.
“Did Pfizer assess myocarditis in the clinical trials?”
Myocarditis was tracked. Pfizer said there were no cases in their clinical trial. Which is great, but expected. The clinical trials were not nearly large enough to capture such a rare event. From our “real world” data, an estimated 75-100 mild cases occur among every 1 million young males. So, we need at least 13,000 vaccinated kids to find 1 mild case of myocarditis. This is just not feasible (money and time wise).
Before authorization, the FDA/CDC needs to answer a critical question: For every 1 case of vaccine-induced myocarditis, how many hospitalizations will we prevent and how many lives will we save? Also, how many COVID19-induced myocarditis cases will we prevent? This is an important, hypothetical analysis that will be conducted using the data available to us. And we will get an answer. Just like we did for adolescents. Unfortunately, though, the only way to assess the “true” rate of vaccine-induced myocarditis will be to roll out the vaccine to the public.
“Did clinical trial assess fertility/ puberty?”
No. Well, I doubt it. There are a few reasons why they would not assess this:
There is no biological plausibility. There is no biological reason why we would expect the mRNA vaccine to impact fertility. mRNA degrades within 72 hours and the fat bubbles that carry the mRNA degrades within 4 days. The vaccine doesn’t linger in our body for decades; it just gives the instructions and then leaves.
There is no safety signal. We have more than a 12 months of clinical trial data for the adult COVID19 vaccine. Many women have gotten pregnant after receiving the COVID19 vaccine. Millions of adolescents have also been vaccinated. No safety signal has been detected. We’re assessing the vaccines’ impact on mensural cycles; an immunological response (like a fever or body aches) to the vaccine. But there is no indication this impacts fertility or safety. I will put together a post in the coming weeks.
There’s the unfortunate, long history of us not doing a great job at studying women in research studies. Studying the impact of female biology on vaccines, drugs, or other disease areas remain suboptimal (and borderline unacceptable). There are national initiatives to improve this in research. But not in time for COVID19 clinical trials.
Fertility is a major concern for parents and the COVID19 vaccine. This was the topic of the majority of questions I received. And, it’s completely natural. We, humans, are designed to procreate; this is our biological existence. Concern regarding our kids’ fertility is real.
But we also need to recognize anti-vaxxer movements, which drive mis/disinformation online, take advantage of this fear. We’ve seen this over and over again with other vaccines (HPV, Hep B,…). In fact, some developing countries still have polio because of the widespread fear that polio vaccines cause infertility. Expect that infertility misinformation will circulate in the weeks to come.
“We’ve seen the benefit of vaccinated adults with regard to hospitalization and death. Do we expect the same sort of protection for kids?”
Yes, we absolutely expect these vaccines to help with mild, moderate, and severe disease for kids. In fact, we hope vaccines to work even better than for adults, like we saw with the adolescent vaccine. We also expect vaccines to prevent long COVID and reduce transmission, just like we see with adults.
“Do we know if kids will need a booster or is that TBD?”
TBD. Let’s try and get this first shot first!
“Once fully vaccinated, do kiddos still need to wear masks? (My daughter is interested in this one!)”
It depends what perspective we want. From an individual-level perspective, kids will be as protected as they can after the vaccine. Parents (or schools) can use this as an incentive if need be.
From a population-level perspective, if enough kids (and adults) get the vaccine, this virus will finally calm down in the United States. And, yes, at that point, kids won’t have to wear masks if they don’t want to. Getting a vaccine will 100% help us get to that point. It continues to be a team effort.
“Will vaccinating 5-11 year olds actually help ‘end the pandemic’?”
Yes. It’ll help with pediatric hospitalization rates. It will help with transmission, and thus it will help with adult hospitalization rates too.
According to Kaiser Family Foundation poll, 1 in 4 parents will vaccinate their 5-11 year olds today. So, that means about 7 million kids will get the vaccine right away (2% of the total population). 2 in 4 parents will wait and see. Will this help end the pandemic? Absolutely. It won’t be the silver bullet, but it will be a step in the right direction. At this point, anything helps.
“My daughter is 4 years old but the same weight as a 5 year old. Can she get this vaccine?”
The vaccine dosage isn’t based on weight. It’s based on the maturity of a immune system. So, it’s best if you wait (unfortunately) for the right dosage. At the very least, please have a discussion with your pediatrician.
“Any news on the 6 month-4 year old vaccines?”
In Pfizer’s press release yesterday, we got a big hint: “(Data) for the other two age cohorts from the trial – children 2-5 years of age and children 6 months to 2 years of age – are expected as soon as the fourth quarter of this year”. So maybe Christmas 2021? But a lot has to go right before then. The press release also made it clear that this vaccine has an even smaller dose (3 mcg).
“Where is the Moderna shot for kids?”
I have no idea what’s happening with this. I was hoping we would have it soon, too.
Hope this is helpful! These next few weeks/months will be crucial for our children. Hang in there, YLE
More questions? I’m SO excited to join Dr. Emily Oster (if you’re a parent, you know) for a live briefing and Q&A: “Navigating the Pandemic with Kids.” We’ll answer questions about the vaccine as well as back-to-school safety and risk trade-offs. This event will be moderated by the amazing Christina Farr (health reporter for CNBC). It will take place on September 28 at 1:30pm ET. Register here: https://lu.ma/livebriefing to join, submit questions, (or to receive the recording afterwards).