The vaccine got to us incredibly fast
Americans got their vaccine in 9 months. This shatters all records of previous vaccines. (Before the pandemic, the fastest vaccine was made in 4 years). But it doesn’t mean scientists were rushed. Scientists had a few key things on their side that helped speed up the timeline without sacrificing quality.
Previous research.
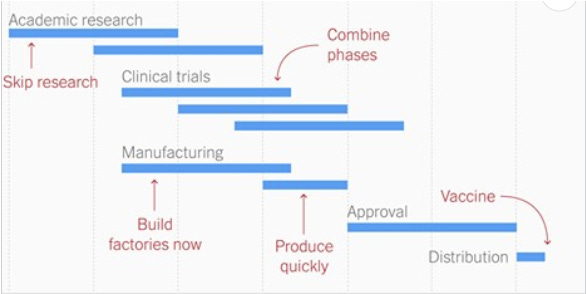
We relied heavily on our previous SARS and MERS work. We were able to do this because COVID19 is roughly 80% identical to SARS. In 2003, we created a vaccine to recognize the SARS’ spike and started moving through clinical trials. However, trials ended because the SARS epidemic faded off on its own, and thus money dried up. If money didn’t dry up, we could've had a COVID19 vaccine much quicker. However, this also meant that we didn’t have to start from scratch in 2020. We were able to skip the pre-clinical and academic research (which takes years) because we were already very familiar with spike proteins, coronaviruses, and vaccines. Also, because of this SARS work, pilot factories were already planned to produce enough vaccines for trials. This groundwork was paramount to getting the vaccine trials up and running in March 2020.
2. Money and resources.
Never, in the history of public health, have we had this much money and this many people working on one disease at one time. Normally, researchers need years to secure funding, get approvals, and study results piece by piece. For example, researchers are typically dependent on grants. In order to get grants, you need data and months (if not years) go by between Phase I, II, and III in order to get data to secure money. But the pandemic is not “typical”. Scientists didn’t need to do this because of the monetary support from the federal government. They were able to just go.
3. High levels of disease.
The FDA specifies, before trials, the efficacy threshold needed to be considered for emergency use. For COVID19, companies needed a higher than 50% efficacy rate. In order to get to that threshold, trials needed to drag on until enough people got infected with COVID19. For example, in Phase III of Pfizer, scientists needed 162 people to get COVID19. Then they could compare how many of these 162 people were in the vaccine group and how many were in the placebo group. COVID19 transmission is rampant in the United States. They needed less people to enroll in the study to reach this threshold. This isn’t always the case in RCTs.
4. Production.
Sponsors were able to start producing the vaccine WHILE the vaccines were currently in trial. This is also very unusual. They were able to do that because governments, around the globe, were willing to put bets on the vaccines (risk vs. reward). Building and manufacturing early shaved off a ton of time, as the vaccine sponsors could anticipate that factories would be useful for a future vaccine.
5. Overlapping phases.
The scientists didn’t wait for Phase I to complete before moving onto Phase II. And didn’t wait for Phase II to end before moving onto Phase III. This isn’t the first time in history this has been done. In fact, this type of design even had a name before the pandemic: seamless and adaptive design. We basically save a ton of time by removing the “white space” between phases. For example, when we combine Phase II and Phase III, scientists evaluate “dose selection” (which is typically determined in Phase IIb) and “confirmation” (typically Phase III) into one trial. There’s quite a few advantages to this type of design (in addition to saving time). First, there’s more efficacy/dose information prior to triggering Phase III. Also, there’s higher chance of patients within the trial to be treated with efficacious and safe doses.
6. Enrollment
As a researcher, it’s incredibly difficult to find people to volunteer for studies. One follower also mentioned this: “I manage trials and my current study aims to find 240 subjects in TWO years”. The amount of people who flooded to volunteer for these studies is incredible. This couldn’t have been done without each and everyone of them.
Bottom line: Speed does not mean rushed. It meant, in this case, leveraging a whole lot of smart people, money, and decades of previous work to get us a vaccine in 9 months.
Love, YLE
Data sources:
More on seamless design: https://pubmed.ncbi.nlm.nih.gov/20724313/
More on timeline (with cool graphics to play around with): https://www.nytimes.com/interactive/2020/04/30/opinion/coronavirus-covid-vaccine.html