Public health touches all aspects of our lives, not just during a pandemic. Thanks to your feedback, this newsletter will continue with Covid-19 updates and address other public health topics, too. To choose what topics land in your inbox, click HERE.
This week the Supreme Court heard a case about access to the first pill used in most medication abortions in the U.S.: mifepristone. Their decision should come down in June or July.
At the center of this case, the plaintiff argued that the FDA ignored safety concerns when it eased restrictions on the use of mifepristone. The defendants relied heavily on a few studies that claimed the abortion pill was unsafe.
However, in a stunning turn of events, the publisher of these scientific studies (SAGE) retracted them last month due to methodological and ethical concerns.
Why were these retracted? (What does that even mean?) And what are the implications?
Retractions are very rare
A retraction is the removal of a published article from a scientific journal. This is a big deal as it doesn’t happen because of some small error. A retraction can be done by the authors (realization of a huge error) or by the publisher (over ethical concerns, fraud, plagiarism, etc.).
It’s unusual for a scientific paper to be retracted (about 1 in 1000), but the rate is increasing. Misconduct accounts for the majority of retractions.
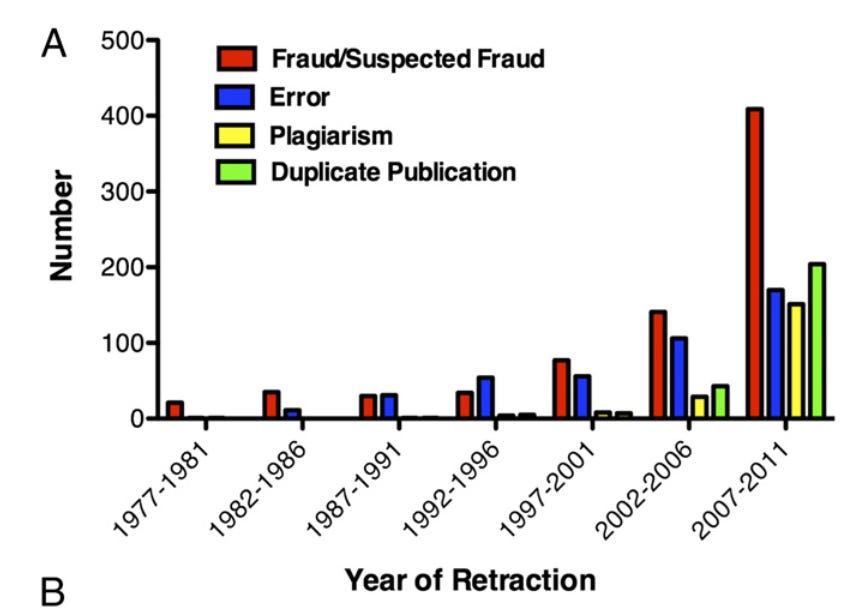
Behind the abortion study retractions
The two studies used in this week's Supreme Court case were peer-reviewed and published in 2021. After publication, though, other scientists started voicing major concerns about the statistical methods (and thus questioning the conclusions).
One external group took a deep dive and published their concerns in a pre-print in another journal. They found:
The codes used to define “abortion-related” emergency room visits were inaccurate. For example, the study used medical codes for ectopic pregnancies that naturally occurred, not caused by abortions.
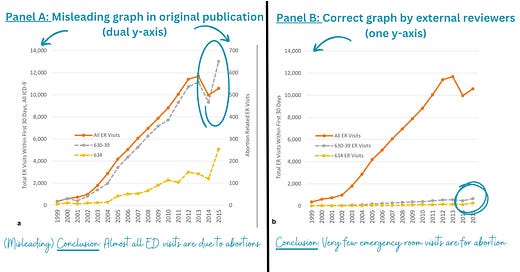
Findings were presented in a deceiving way, like using dual y-axes on one graph. The left panel on the figure below is what was published. It shows abortions are leading to a lot of emergency room visits. However, when the y-axis is displayed properly on the same scale, abortions lead to a very small number of ED visits.
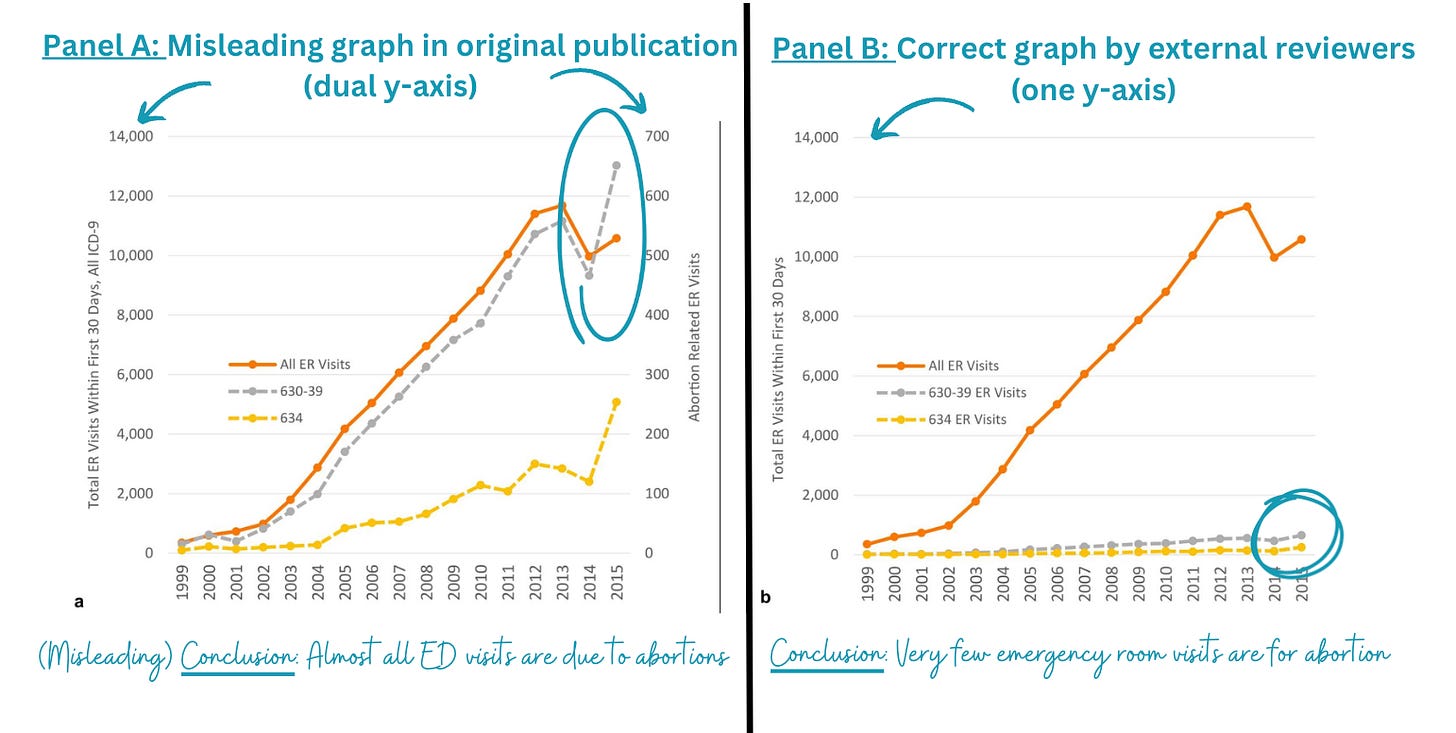
After these concerns were raised, SAGE asked for a review of the articles post-publication. They had two subject matter experts and one independent statistical reviewer take a look. Then SAGE retracted the paper based on three major factors:
Methodological and statistical concerns. Specifically, “unjustified or incorrect factual assumptions,” “material errors,” and “misleading presentations” of data that “demonstrate a lack of scientific rigor and invalidate the authors’ conclusions in whole or in part.”
Ethical considerations. The authors were members of three pro-life advocacy organizations, despite declaring no conflicts of interest in the study.
More ethical considerations. The peer reviewer (who is supposed to be an unbiased third party) didn’t disclose their conflict of interest— that they know the authors personally.
SAGE did not publish the experts’ peer review, which is normal practice. However, given the stakes of this case, it could be coming.
Implications can be long-lasting
Clinical guidance and policy are (ideally) built on decades of research and consideration of the totality of evidence. In the case of mifepristone, more than 100 studies show it’s safe—in fact, safer than Tylenol—with only a few dissenting studies. Some dissenting studies have now been retracted due to methodological and ethical issues.
However, big mistakes can make it past the peer-review process, and, in some rare cases, “mistakes” are intentional and egregious. Even if studies are retracted, they can do a lot of harm (just look at the Wakefield study on autism and the MMR, for example).
Bottom line
A lot of emotion and opinions surround abortion. But I hope we can all agree that we need a solid foundation of data to make smart policy decisions. This bedrock is highly dependent on ethical scientists and a strong review process.
It’s shocking that these studies reached the Supreme Court and were used as a source for decision-making.
Love, YLE and HM
Heidi Moseson, PhD, MPH is a reproductive epidemiologist and scientist at Ibis Reproductive Health. She studies abortion access in the U.S. with a particular focus on self-managed abortion with medications.
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, M.P.H. Ph.D.—an epidemiologist, wife, and mom of two little girls. During the day, she is a senior scientific consultant to several organizations, including CDC. At night, she writes this newsletter. Her main goal is to “translate” the ever-evolving public health world so that people will be well-equipped to make evidence-based decisions. This newsletter is free, thanks to the generous support of fellow YLE community members. To support this effort, subscribe below:





Mifepristone is on the WHO'S List of Essential Medicines. It's use mainly to treat several indifferent auto-immune disorders. There are millions of peoole who take this as a life saving medication on a daily basis. This lawsuit is putting a lot of vulnerable people at risk for political gain that's based in large part in religious beliefs which is also unconstitutional. 11 states have a State Constitutional right to privacy that would make anti-abortion laws unconstitutional on a state level in those states. The Supreme Court shouldn't be hearing this case at all. Our institutions are failing us daily. No one had an issue with mifepristone or it's FDA authorization until the Christofascists got their claws into the GOP. This is bigger than just one medication or abortion. This has major implications for our rights across the board. Remember this when I comes time to vote. Millions of women and girls are counting on us to make this right.
Can we make misuse of health statistics to propagate fallacies a felony? The impact of such actions is far greater than the bodily injury-type felonies that affect one person at a time.