Si quiere leer la versión en español, pulse aquí.
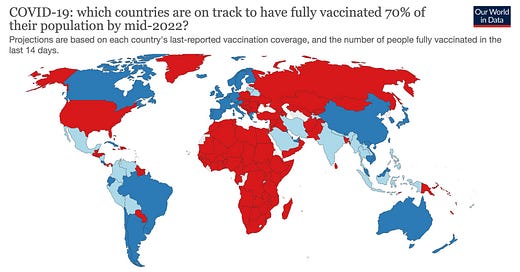
One way (maybe the only way) we’re going to get out of this pandemic is to vaccinate a large portion of the global population. To the WHO, this means reaching 70% of the population by mid-2022, which is ~3 billion unvaccinated people with 6-9 billion doses before another variant of concern.
A diverse portfolio of vaccines that utilizes a number of different biotechnologies is of critical importance. While mRNA vaccines are innovative and effective, they pose logistical storage challenges to reach remote communities. The mRNA pharmaceutical companies are also not sharing their vaccine patent, which doesn’t allow others to manufacture. In addition, a diverse portfolio of vaccines frees up supply bottlenecks, provides options for those allergic to vaccines ingredients, and, among vaccines that use more traditional biotechnologies, will reduce vaccine hesitancy.
Two new vaccines have been added to our global repertoire: NVX-CoV2373 and CORBEVAX. These will be nothing short of game changers for the pandemic. Here is their story and how they work:
NVX-CoV2373
NVX-CoV2373 was created by Novavax, a small pharmaceutical company from Maryland. Before the pandemic they almost lost it all, but made a huge comeback after Operation Warp Speed took a chance on them. (I recommend reading their history over coffee; it’s fascinating). This will be their first vaccine to make it to the market.
Novavax is using a different vaccine biotechnology from other COVID19 vaccines. It contains the coronavirus spike protein combined with an immune-boosting compound from the soapbark tree. Once the immune system encounters the spike protein (which is harmless alone), the body produces antibodies against SARS-CoV-2 and thus protect from future infection.
This method has a much longer track record than the newer approaches, as it’s used for some flu and HPV vaccines. The exciting (and innovative) aspect of this vaccine is that Novavax found a way to make it in moth cells (rather than mammal cells). The moth cells become little factories that pump out coronavirus proteins. The scientists then extract and purify the spike proteins for vaccines. This allows Novavax to manufacture the vaccine much quicker than other vaccine types.
Phase III clinical trials (called PREVENT-19) were released in 2021 and results were published in the New England Journal of Medicine. NVX-CoV2373 had an efficacy of 100% against moderate-to-severe disease with the original variant and 90% efficacy overall. Which is nothing short of fantastic. Since, the vaccine has also been shown to be highly effective against other variants of concern, like Delta, Beta, and Omicron.
However, it’s taken months for Novavax to submit an EUA application to the FDA because they ran into some production issues. When a company applies to the FDA, they include data about the safety and effectiveness (which we all focus on), but they also submit data on manufacturing and production. And Novavax had a hard time passing production purity tests—the FDA requires vaccines to consistently pass a purity of 90% so contaminants don’t make the vaccine less effective or potentially cause some people to adversely react. Last October, Politico reported that Novavax was showing purity levels hovering around 70%.
This week Novavax announced they’re applying for an EUA in the United States—a signal that they refined their production to consistently pass purity tests for the FDA. Novavax has already been approved in several other countries, including India, South Africa, Australia and the E.U. On December 17, the WHO issued an emergency use listing (EUL) so Novavax could give a much-needed boost to COVAX—an international consortium of vaccine supply—to vaccinate more people in lower-income countries.
CORBEVAX
The second game changer vaccine is called CORBEVAX; in fact it’s been dubbed the “The World’s COVID-19 Vaccine.” This work was not led by a big (or small) pharmaceutical company but by two scientists at Texas Children’s Hospital and Baylor College of Medicine: Drs. Maria Elena Bottazzi and Peter Hotez. The two have been working together on coronavirus vaccines for the past two decades, including the development of a SARS vaccine in 2003. So when the pandemic hit, they were able to quickly pivot to COVID19. They were not funded through Operation Warp Speed. In fact, they had a very difficult time receiving funding from the government so they turned to philanthropic support, including from Tito’s Vodka.
CORBEVAX is also protein based, but uses a yeast fermentation method that’s been around for several decades and doesn’t use human or animal cells. In fact, this is the same biotechnology used to make the Hepatitis B vaccine, which millions of us already have.
CORBEVAX completed Phase III clinical trials in India, which involved more than 3,000 participants. The vaccine was found to be highly effective against the original virus, with a >90% vaccine effectiveness and 80% effective against Delta. The clinical trials showed it was also safe and well tolerated, as it had 50% fewer adverse events than other vaccines. As NPR reported, one drawback to this biotechnology is that it can't be modified as quickly as mRNA vaccines for new variants.
Because this vaccine biotechnology has been around for a long time and because Drs. Hotez and Bottazzi made the intellectual property available to everybody, vaccine producers all around the world can make it. In fact, CORBEVAX is already licensed to four vaccine producers: Biological E in India, Biopharma in Indonesia, Septa in Bangladesh, and ImmunityBio in South Africa and Botswana. On December 28, it was authorized for emergency use in India. And, two days ago, Drs. Hotez and Bottazzi were nominated for the Nobel Peace Prize.
Bottom line: The underdogs are pulling through! And we should all be cheering as these add diversity to our vaccine portfolio, which will get us one step (or more like leaps) closer to ending the pandemic.
Love, YLE
I have no conflicts of interest to report with any of these vaccine sponsors.
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, professor, researcher, wife, and mom of two little girls. During the day she has a research lab and teaches graduate-level courses, but at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here:



Nice to see moths doing something other than putting holes in my knitting yearn. Nice reporting job....Bon chance to Tito's Vodka as well.
Sorry, just a retired Micrrobiologists humor. You are giving us wonderful information.
What are the chances of these two being offered within the US? I know there are some that do not like the idea of the RNA vaccines. Also, think these would eventually make great boosters as they work on a different part of the immune system.