Yesterday, VRBPAC— FDA’s external scientific committee—met to discuss the latest clinical trial data for RSV vaccines. This is a big deal because it could be the first RSV vaccine to market— something we’ve been waiting on for decades. (Literally since the 1960s, with the first RSV vaccine candidates ending in terrible failure among kids.)
The purpose of this meeting was to discuss the safety and efficacy of the vaccines. Some of the safety data was tight, so keep this post in your back pocket. We smell misinformation brewing.
Here are your Cliff notes.
Vaccine options
Both Pfizer and GlaxoSmithKline (GSK) are applying for licenses. Their RSV vaccines are for adults over the age of 60. Both are one dose vaccines given in preparation for winter season.
The vaccines differ slightly in their design:
GSK (vaccine name: Arexvy) uses an adjuvant (called AS01E)—an ingredient that helps the vaccine create a stronger immune response and is shown to give longer immune responses.
Pfizer (vaccine name: Abrysvo) has no adjuvant. Everyone has already had an RSV infection in their life, so by just giving the protein, they can activate memory cells from previous infections to do all the work. Adjuvants usually give more symptoms too, so including one was not worthwhile in Pfizer’s judgment. (Pfizer didn’t see any benefit when it examined adjuvants).
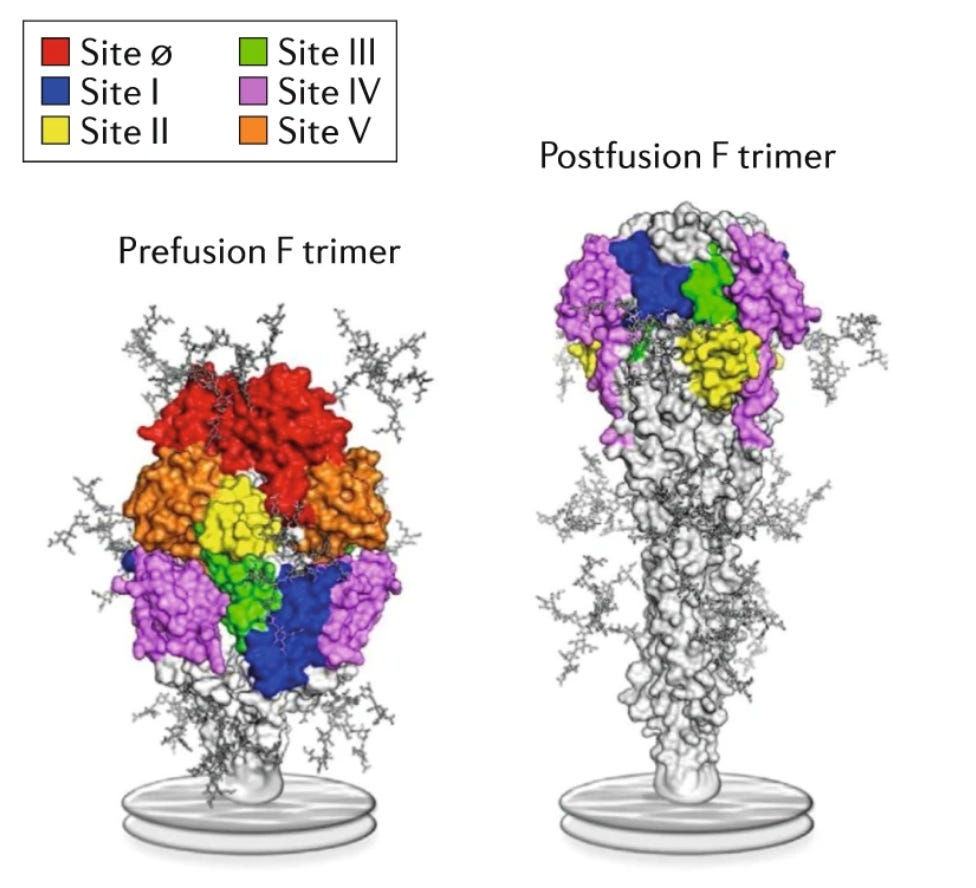
Both of these vaccines target the “F protein.” This is similar to the spike protein of coronaviruses— the key into our cells. The problem is that RSV’s F protein is extremely unstable— it shifts between two “shapes” (prefusion and postfusion; see picture above). The vaccines induce antibodies specifically to the orange and red areas found only on prefusion shape. The absence of targeting these colors is thought to be a major reason the first RSV vaccines failed.
Safety
The companies tested the side effects of the vaccines by themselves, co-administered with flu vaccine, and after flu vaccine. The latter two situations are more likely close to reality in the Fall. They did not test the co-administration with COVID-19 vaccine (it’s unclear why, but those studies will be done).
Side effects immediately after the dose, like fever and body aches, were not common. This is a welcome reprieve from the COVID-19 vaccines.
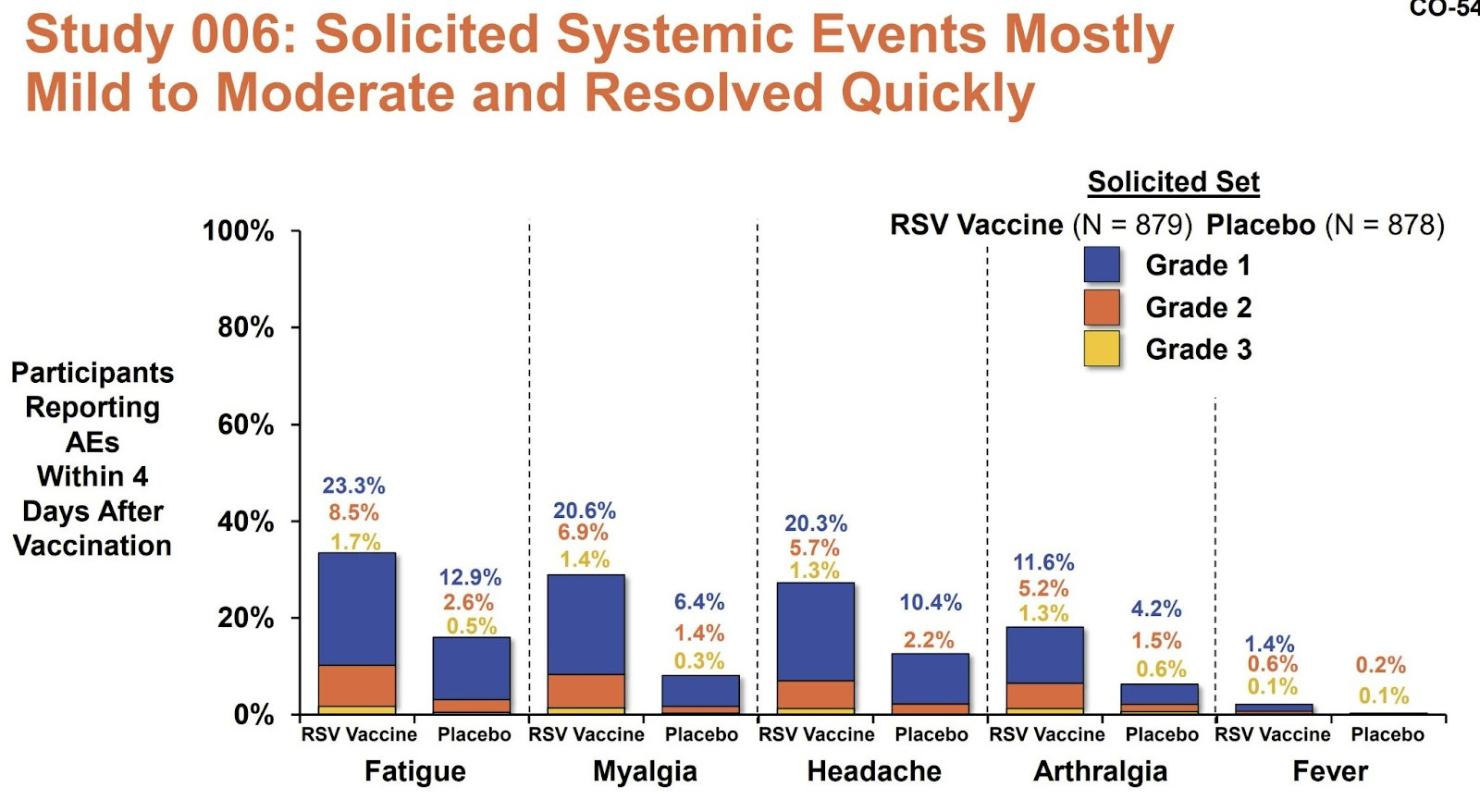
Severe adverse events were rare and similar between vaccine and placebo groups. However, there were two potentially concerning signals:
Acute disseminated encephalomyelitis (ADEM)—a neurological condition in which the brain or spinal cord rapidly swells due to inflammation.
GSK: 2 cases in participants who got the vaccine at the same time among 890 individuals. Pooling their trials, this is a rate of 2 events per 15,000.
Background rate: 1 to 2 cases of ADEM per 250,000 people per year.
Guillain-Barré syndrome (GBS)—also a nerve condition caused by inflammation that causes weakness and paralysis that typically begins in the legs and rises.
GSK: 1 case for a total incidence of about 1 per 15,000.
Pfizer: 2 cases for an incidence of about 1 per 9000.
Background rate: 1.5 to 3 per 100,000 people per year.
It’s concerning that both vaccines produced similar adverse events and at similar rates that are, in an absolute sense, still well above their background rates.
Efficacy
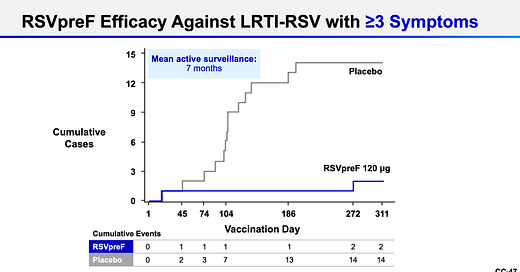
How well do they work against severe disease? This was defined as the number of lower respiratory symptoms (like wheezing, needing to breathe more quickly, or needing supplemental oxygen).

The vaccines overall had great efficacy:
GSK: 82% efficacy (7 events in the vaccine group and 40 in the placebo group).
Pfizer: 86% efficacy (2 events in vaccine group and 12 in placebo group). They also measured less severe symptoms, which equated to a 66% efficacy (11 in vaccine group and 33 in placebo).
This efficacy held across ages, across severity of symptoms, and across RSV strains. There weren’t many participants with comorbidities, though, so the efficacy for them was around the same, but with less certainty for how this will translate to the real world.
Additionally, the absolute number of events that occurred was small (especially with Pfizer’s vaccine), which means there is greater uncertainty about the efficacy. However, a human challenge study in healthy 18- to 50-year-olds who received Pfizer’s vaccine showed excellent results, which suggests that these measures (although in older adults) are likely valid.
Discussion
The main source of hesitation from this committee came from adverse events, especially ADEM and GBS. Because the number of events is so small, it’s hard to know whether these are coincidental events or reflect a real safety issue. But, it would be tough to study with a randomized controlled trial because many people would need to be enrolled to see safety signals.
One member mentioned the worry about public messaging around these vaccines against a backdrop of misinformation. The chair even said: “We have a vaccine program to worry about, not just the pathogen.”
The committee made a case that the vaccines should be rolled out to the public with enhanced safety monitoring for these conditions to define how much of an increased risk there is and then reevaluate.
Durability. The durability of the GSK vaccine was measured by testing antibody titer over time and age group; as expected, it decreased over time but still remained elevated compared to pre-vaccine levels. T cell responses showed a similar pattern.
However, what this means for protection against severe disease in humans is not currently known. This is particularly a concern with RSV because we know that infection-induced immunity is incomplete.
The clinical trials were too short to track performance of the vaccine across seasons. However, the companies are collecting more data to understand this better.
Some advisory committee members felt that because RSV is not currently a public health emergency in this age group, it would be best to conduct trials for additional data before granting licensure.
Vote
The members voted on whether the FDA should continue with these applications. The majority voted yes, but it was a tight vote (given the sources of hesitation above).
What’s next?
The ball is now passed to the FDA. Since the companies are applying for a license, it will likely take a long time (months) to sort through the paperwork. In the meantime, more data will become available that will be closely followed.
Bottom line
It looks like older adults will have another way to protect themselves in Fall 2023, which is a huge milestone for public health. The first RSV vaccines will be a big deal, but we still have questions about how they are best used. Future data will help.
Love, YLE and Edward Nirenberg
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, data scientist, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank and is a senior scientific consultant to a number of organizations, including the CDC. At night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support this effort, subscribe below:





Very grateful to Dr. Jetelina, once again, for the report on this. Have to say, as one in the target group, this is the first time where I find I am hesitant to get a vax, at least until more is known. I thought there were really good cautionary comments coming out of some members of the committee.
>The level of incidence of adverse events gives me pause, for one, and I’m not too keen on the potential duration of side effects—a 20%+ chance of four days of fatigue or myalgia post-vax is not high on my hit parade (though here I may not understand the charts).
>I was also struck by this: “Some advisory committee members felt that because RSV is not currently a public health emergency in this age group, it would be best to conduct trials for additional data before granting licensure.”
>Information on durability also seems suboptimal, per Dr. Jetelina’s comment in that section, “However, what this means for protection against severe disease in humans is not currently known. This is particularly a concern with RSV because we know that infection-induced immunity is incomplete.”
So far, I find myself in the camp of the dissenting votes. Of course there are several more months before the vaccines will be offered, so perhaps we will know enough more by which to make a judgment.
Will be interested in the thoughts of others on these issues.
Concur with Susan. Anyone who has read this newsletter for a significant time knows I am a Covid maximalist, fearing (by temperament) the virus and what it can do. Note the use of the present tense. That same fearfulness makes me loath to take a vaccination that has even a 1 in 15,000 chance of getting GBS. I don't think the outcomes of getting RSV for lack of vaccination, are worse than the total catastrophe GBS would be.