Vaccine Update
We cannot vaccinate fast enough. To our advantage, though, many more vaccines are coming down the pipeline. Janssen and AstraZeneca will likely be available next, followed by Novavax which is about to start its research trial.
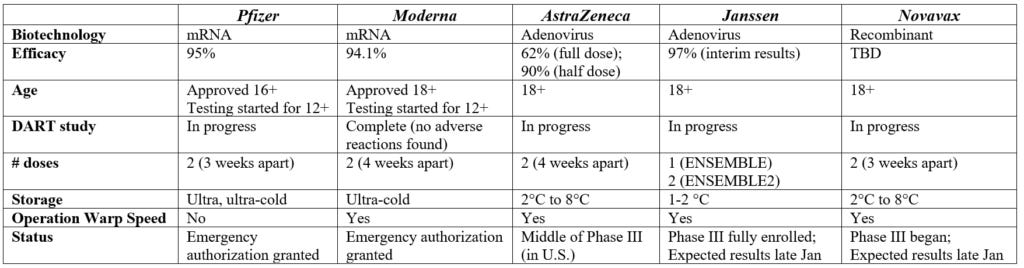
If you’re like me, these are starting to get difficult to keep track of, so I started a Table for myself. It may (or may not) be helpful for you too. It's not very pretty, but I'm a researcher not a graphic designer :)
Over the next few days, I’ll give you updates on each one of these. Today… Janssen.
- Janssen is the pharmaceutical arm of Johnson & Johnson (J&J) company, which resides in Belgium
-Currently in Phase 3 testing a single dose. The sponsor has given this study a name: ENSEMBLE
-Trial is only among adults 18+ with controlled comorbidities. 45,000 people are currently enrolled
-This is an adenovirus-based vaccine; basically a small piece of genetic material from the virus is used to trigger an immune response. J&J has been working on this biotechnology for decades. They just had an Ebola vaccine approved using this same idea. They are currently in trials for HIV and Zika vaccines too. The AstraZeneca also uses adenovirus technology
-Trials were temporarily delayed for an unexplained illness on October 12, but has since determined the event was not related to the vaccine and the trial was safe to resume.
-This vaccine is more rugged than Moderna or Pfizer. DNA is not as fragile as RNA. And this vaccine has a tough coat protecting the pieces. As a result, storage is much easier for the J&J vaccine
-J&J should have safety and efficacy data by end of January. And expected to seek emergency use authorization by early February
-J&J also announced a separate double dose study (ENSEMBLE2)
Love, YLE