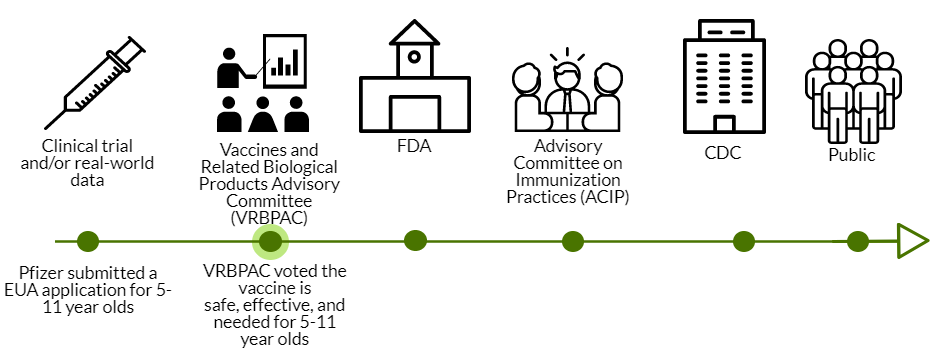
Today VRBPAC (an external scientific advisory committee to the FDA) voted in favor of the Pfizer COVID19 vaccine for 5-11 year olds. VRBPAC was the second stop in a long process to get the vaccine authorized for emergency use.
This was a much anticipated meeting for two reasons:
Originally, the FDA hinted they would not consider an EUA for kids <12 years. But, Delta and pandemic resurgence caused the FDA to change perspectives.
The VRBPAC step was not conducted for the adolescent Pfizer vaccine; VRBPAC doesn’t have to be called for an EUA amendment. But they were called for the vaccine for 5-11 year olds.
So, today 18 members of VRBPAC met to discuss hundreds of pages of data. These members are a mix of pediatricians, immunologists, virologists, epidemiologists, and other scientists across the nation. There were also presentations from the sponsor (Pfizer), FDA, and the CDC. Here was the agenda. Here are all the powerpoints. And here are your cliff notes…
Need
Kids aren’t spared from the harm of COVID19. CDC presented the epidemiology of COVID19 outcomes among 5-11 year olds. These are close to real-time numbers:
Infection: More than 1.9 million 5-11 year olds have been infected by COVID19 during the pandemic. During Delta, there was a sharp increase in cases; 5-11 year olds represented 10.6% of cases in the week of Oct 10 (they make up 8.7% of the population).
There are still kids susceptible to COVID19. Only 42% of kids aged 5-11 years have antibodies from natural immunity.
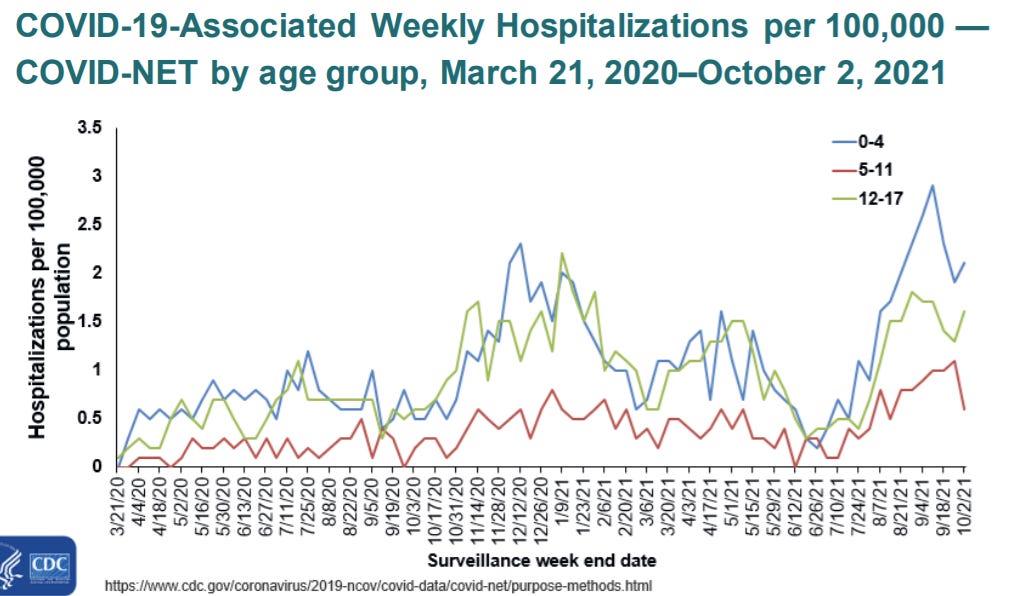
Hospitalization: There’ve been >8300 COVID19 hospitalizations of 5-11 year olds
Over 30% of 5-11 year olds hospitalized did not have an underlying condition.
When compared to other kids, 5-11 year olds had the least number of hospitalizations. But, starting in August, they had the highest rate since the beginning of the pandemic.
Once hospitalized, 1/3 of kids ended up in the ICU.
There were exceedingly low (only 9) hospitalizations for flu during 2020-2021. At the same time, there were significantly higher COVID19 hospitalizations. Had mitigation measures (masks, closed schools) not been in place, these numbers would have been much higher.
MIS-C (multisystem inflammatory syndrome in children) is highest among 5-11 year olds. This is a condition where different body parts can become inflamed, including the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal organs. There’s been 5,217 MIS-C cases reported as of October 4, 2021. 60-70% of patients are admitted to ICU and 1-2% died.
Death: There have been 94 5-11 year olds that have died from COVID19. For context, this places COVID19 as the 8th leading cause of death for this age group. More recently (during Delta), COVID19 jumped to the 6th leading cause of death in this age group.
Long COVID19: 7-8% of kids experience long COVID19. The following symptoms occur among kids for more than 4 weeks: fatigue, headache, insomnia, trouble concentrating, muscle and joint pain, and cough. There are also impacts on quality of life: Limitations of physical activity, feeling distressed about symptoms, mental health challenges, decreased school attendance/participation.
Secondary outcomes
In-person school: COVID-19 in children leads to lost in-person learning and other adverse outcomes. This has resulted in 2,074 schools closed, 1,069,116 of students and 68,718 teachers affected.
Transmission: Kids also significantly contribute to the spread of the virus. Secondary transmission from young school age children can and does occur in both household and school settings
Clinical Trial Results
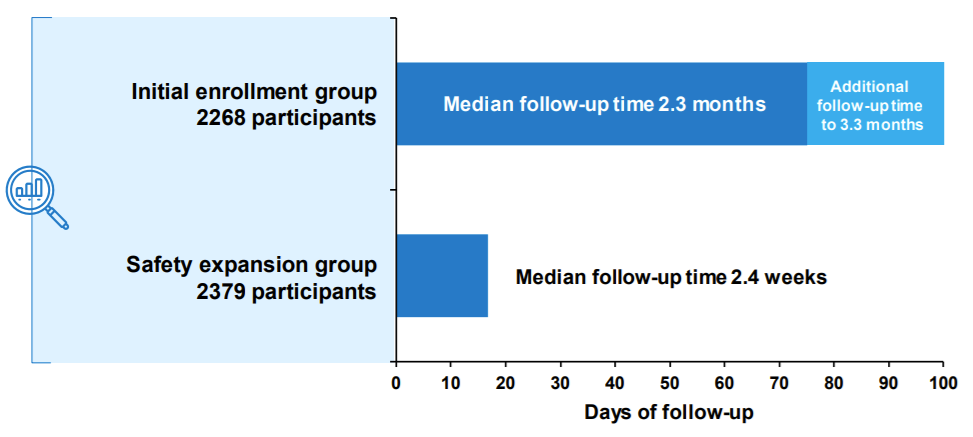
The clinical trial was organized into two cohorts:
2,268 trial participants (including 1,518 vaccine recipients) followed for at least two months past the 2nd dose
A “safety expansion” group of an additional 2,379 participants (1,500 vaccine recipients) followed for a median of 2.4 weeks after the second dose. This was done per the FDAs request “to allow for more robust assessment of serious adverse events and other adverse events of interest”.
First Pfizer presented their results. Then the FDA presented their results. The FDA always analyzes data themselves to ensure there is no conflict of interest. This is normal practice.
Safety
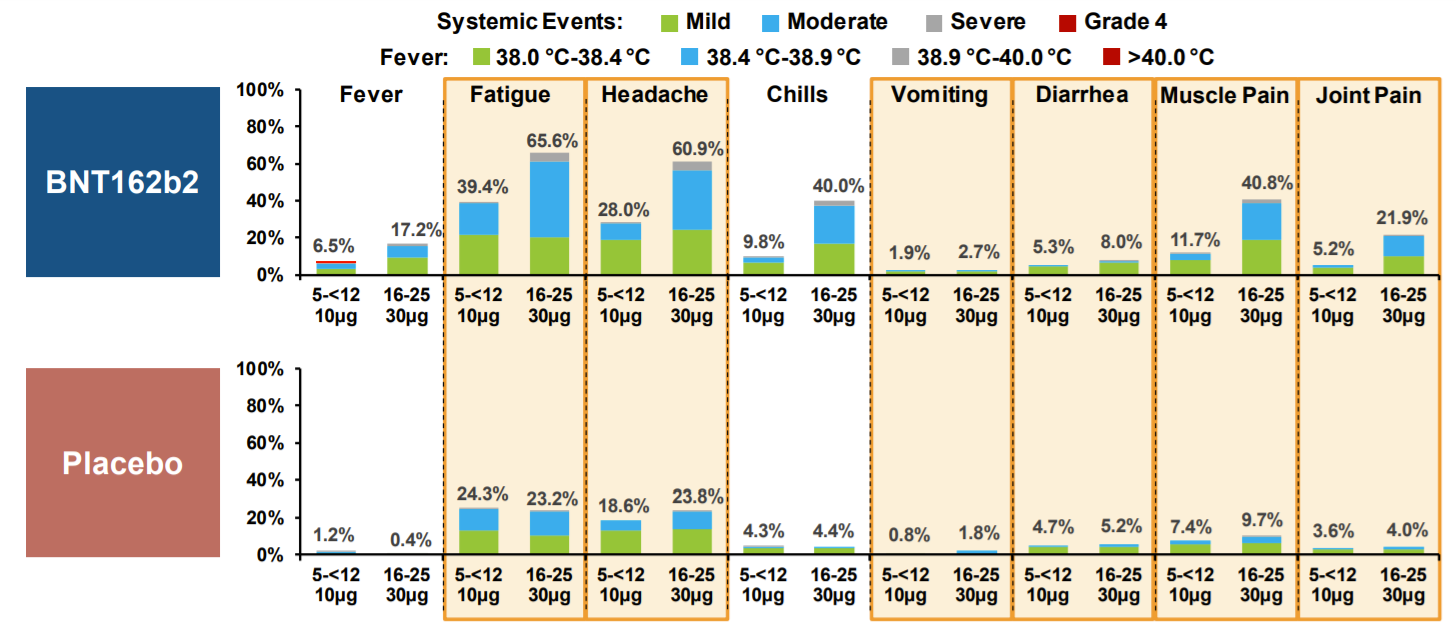
During the clinical trial, the most common adverse events (AEs) was fatigue (39%), followed by headache (28%) and muscle pain (12%).
Most AEs were mild or moderate and resolved 1-2 days after
Interestingly, fever and chills was less frequent compared to older kids/adults
Adverse reactions was higher for dose 2 compared to dose 1
There are two other adverse events linked to the vaccine:
Lymphadenopathy (swelling of lymph nodes): 13 vaccine participants vs. 1 placebo participant. We saw this in adult clinical trials too.
Hypersensitivity: Such as rash and dermatitis after the vaccine compared to the placebo
There were 5 severe adverse events. None were linked to the vaccine:
Ingestion of a penny (1 person in the vaccine group)
Fractures (2 people in the vaccine group and 1 in the placebo)
Infective arthritis (one person in vaccine group)
There were no cases of myocarditis (heart inflammation), anaphylaxis or deaths among ages 5-11 in the clinical trials.
Effectiveness
Does the vaccine work for 5-11 year olds? The FDA required Pfizer to prove immunobridging. Pfizer also included data on two other outcomes:
Immunobridging: This is a process that compares antibodies among 5-11 year olds to another age group (in this case 16-25 year olds) in which the efficacy of a vaccine is already established. The clinical trial found that antibody numbers were comparable to the older age group. In other words, the vaccine works.
Effectiveness against Delta: Pfizer also included data on a subsample of participants’ (34 participants) to assess the effectiveness of the vaccine-induced antibodies on specific variants. The vaccine worked great against Delta.
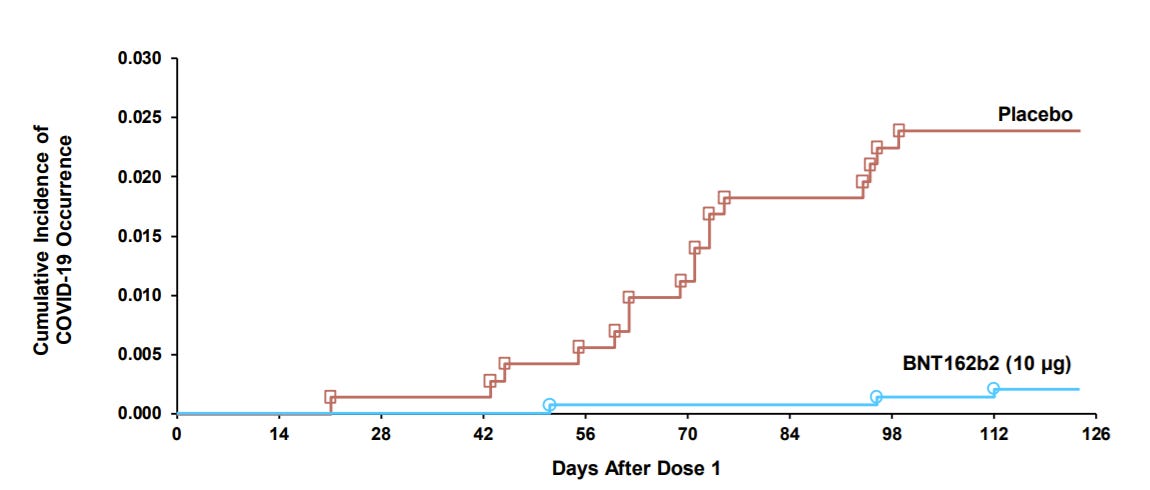
COVID19 disease: Pfizer also showed efficacy. During the clinical trial there were 19 cases of COVID19: 3 cases in the vaccine group and 16 cases in the placebo group. This equates to a 90.7% efficacy.
Myocarditis
No myocarditis cases were reported in the clinical trial. This is great news but expected. The clinical trials were not nearly large enough to capture such a rare event.
Myocarditis is the principal concern that people have with mRNA vaccines with children. So, CDC presented everything we know about vaccine-induced myocarditis. Key take home points:
Myocarditis is a true safety signal, but rare. There have been 877 cases of vaccine-induced myocarditis among 12-29 year olds (out of more than 100,000,000 vaccinated). Of these, 829 were hospitalized and 77% recovered. At the time of analysis, 5 people were in the ICU. No myocarditis cases reported and investigated by the CDC have resulted in death.
Not all myocarditis should be treated the same. Classic myocarditis (opposed to vaccine-induced myocarditis) has a relatively high mortality rate and can even result in sudden death. Classic myocarditis also impacts on how well the heart pumps blood (called ejection fraction). We don’t see mortality or ejection fraction reduction with vaccine-induced myocarditis. It’s a much more mild form of disease.
Long term effects of vaccine-induced myocarditis. Kids tend to bounce back very well. A key study followed a subset of adolescents with vaccine-induced myocarditis. Adolescents fully recovered from symptoms and arrhythmias ~35 days. Data is limited, but continues to be studied. Unfortunately we are at the mercy of time.
Why is this happening? We don’t know yet. Only 2 of the myocarditis cases among children have been biopsied. We think genetics and hormones may play a role.
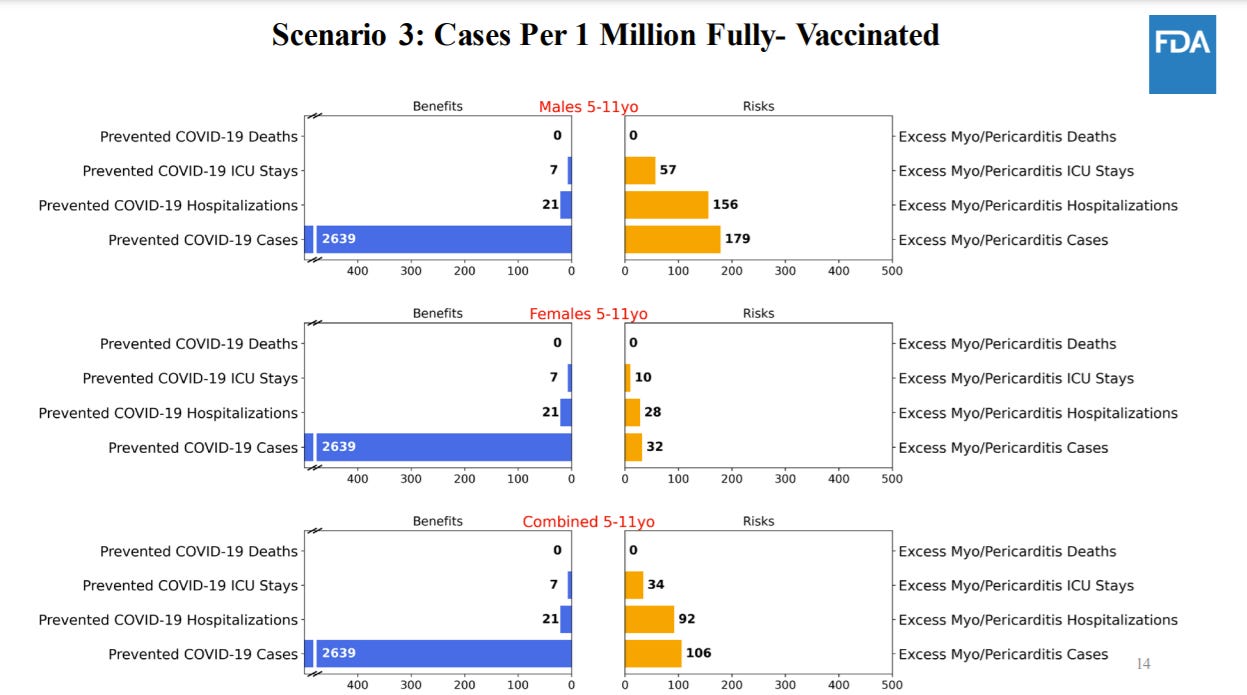
The FDA conducted an extensive benefit/risk assessment through a series of six models. Because we can’t see into the future, biostatisticians ran different scenarios based on varying three variables: COVID19 cases (pandemic could get worse or better); real world effectiveness of vaccines (we think this will be high, but could vary depending on waning and variants); and rate of vaccine-induced myocarditis among this age group (we think it will be lower than adolescents but we don’t know). All of these risks are deliberately conservative. In other words, they looked at worse case scenarios for myocarditis risk and vaccine efficacy. This is what they found…
For five scenarios, the benefits of a vaccine clearly outweigh the risks of myocarditis:
Scenario 1: Used number of COVID19 cases on Sept 11, 2021
Scenario 2: Used number of COVID19 cases at the Delta peak (worse case scenario)
Scenario 4: Used a high real-world effectiveness of the vaccine
Scenario 5: Used high COVID-19 death rate
Scenario 6: Used a lower estimate of myocarditis rate than adolescents (this is the likely scenario given the impact of hormones on myocarditis and given classic myocarditis among this group is so low)
There was one scenario (#3) where benefits didn’t outweigh risks. In this model, the FDA used the lowest level of COVID19 cases like we saw in June 2021. Using this, the model predicts more excess hospitalizations due to vaccine-related myocarditis compared to prevented hospitalizations due to COVID-19 in males and in both sexes combined. It’s important to keep in mind that there are benefits to the vaccine beyond hospitalization.
There was quite a bit of discussion around the limitations of the risk/benefit analysis. For 5 to 11 year olds, 18% of COVID19 hospitalizations are not COVID19 related. The FDA risk/benefit ratio didn’t take this into account. The risk/benefit analysis also did not take into account natural immunity protection.
Discussion
As you can imagine, there was a robust discussion among voting members:
Make it available to certain 5-11 year olds? Two members voiced frustration that this was a binary choice: all or nothing vaccine for 5-11 year olds. Some asked whether they could rephrase the question for specific kids, like obese or immunocompromised. Some pointed to the number than 42% of kids have natural protection and that vaccines may not help that much.
On the other hand, others pointed out that 32% of kids hospitalized don’t have a comorbidity, and we have no idea where the pandemic will go. We have a flu vaccine to prevent 100 deaths a year and kids are dying at higher rates from COVID19. And, most importantly, this isn’t a decision for VRBPAC. VRBPAC decides whether the vaccine is safe and effective. Defining the policy (i.e. who gets the vaccine) is ACIP’s job. If VRBPAC doesn’t authorize EUA for kids, then no kid can get the vaccine.
Should 11 year olds wait for the 12 year old dose? This was specifically asked by the members because of the 3 breakthrough cases in the clinical trial: 10, 10 and 11 years old. Pfizer said that the effectiveness of the smaller dose works the same whether you are 5 or 11. They think breakthrough cases were older because they were more exposed to the virus. Pfizer hasn’t tested this dose among 12 year olds. So, ultimately, they don’t know if parents should wait. CDC should weigh into this next week.
Does the vaccine prevent transmission? Pfizer did not assess asymptomatic disease (and thus transmission) in the clinical trials. So, technically, they don’t know. They do know that adult vaccines reduce transmission. But adults had a higher dosage, does this matter? We don’t know, but we hypothesize that pediatric vaccines reduce transmission to some degree.
Vote
So, VRBPAC needed to vote: “Based on the totality of scientific evidence available, do the benefits of the PfizerBioNTech COVID-19 Vaccine when administered as a 2-dose series (10 µg each dose, 3 weeks apart) outweigh its risks for use in children 5-11 years of age?”
Yes: 17 votes
No: 0 votes
Abstain: 1 vote
The ball now goes to the FDA. Then, it goes to the ACIP and CDC next week. If all goes well, vaccines in arms for 5-11 year olds will come at the end of next week.
Love, YLE
I joined the President of the American Academy of Pediatrics last week for a Q&A regarding the vaccine for 5-11 year olds. See the recording here.
Other Q&A questions for parents answered in my newsletter here
Here are common vaccine concerns for parents and how to address them. If you’re a paying subscriber and want the PDF, just reply to this email. The latest draft is for adolescents specifically, but I’m happy to update for 5-11 year olds if helpful.




Thank you! Paid subscriber here. I thought I read somewhere that mRNA vaccines in teens aren’t waining as much as adults, (comparing the same amount of time from vaccination). (Pfizer) Is this true? Thank you! Stay well!
Thank you for your fast follow up. I watched the meeting today and your thoughts always help me understand everything more clearly. Some of that discussion was wonderful and some of it was frustrating to listen to! I am crossing my fingers for these next 3 hurdles and am realllllllllly hoping shots in arms will be a reality soon. Thank you so much for all your work, you are amazing!