Thank you for all of your questions! You’re keeping us busy here at YLE. Here are answers to the top 7 that I’ve received in my inbox.
When will we know about the COVID-19 vaccine?
The ACIP meeting is now scheduled for September 12. This will be a huge meeting, as we will find out:
Who is eligible for a COVID-19 vaccine and why.
Cost-effectiveness, given this vaccine is now privatized. In other words, we will get an answer to the question: Do the benefits of a vaccine outweigh the costs for all age groups?
Updated myocarditis data for younger males (I hope).
I will be in attendance and provide cliff notes.
Then, vaccines should be available mid-to-late September.
Can I get the vaccines (flu, RSV, and/or COVID-19) at once?
There is no combined shot (some companies are working on it, but will not be available for years). This means that, if you’re eligible, you will need three shots to protect against the three viruses this fall.
You can get them all at once. But it may not be ideal. It just depends on your situation and your comfort with unknown risks:
Benefits to getting all at once: Going to the doctor/pharmacy multiple times can be a pain for many people, including grandparents. Or some people may tend to forget to go. Getting all three at once may be the best option in this situation.
Benefits to staggering: The optimal timing of vaccines is different for all three viruses. Also, we don’t know the safety risks of getting all three simultaneously. It hasn’t been studied. In other words, there are unknown risks. This is what we do know:
COVID-19 and flu vaccines are safe together
Older adult RSV and flu vaccines are safe together
Should I wait for the fall COVID-19 vaccine?
I’m telling my family and friends to wait (as opposed to getting last year’s vaccine formula). Of course, there is some risk to waiting, but there are two benefits, too:
While we are in a wave now, we expect a larger wave in winter. Getting it closer to this wave will better prevent infection;
Recent preprint shows that two shots of last year’s vaccine formula resulted in imprinting. This isn’t necessarily dangerous, but it means our antibody factory line (i.e. B-cells) wasn’t updated—it doesn’t broaden protection. Getting an updated vaccine formula will be more helpful against currently circulating variants.
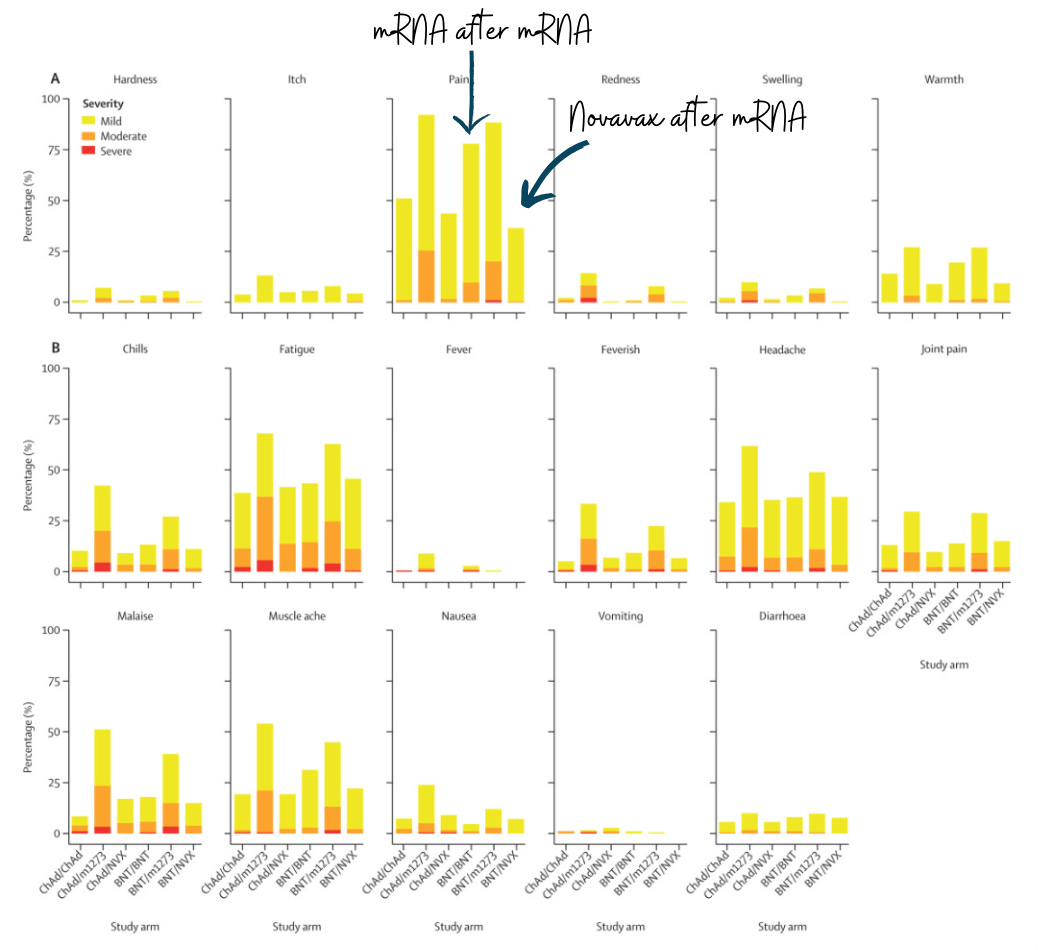
Novavax vs. mRNA COVID-19 vaccine?
Both are great shots. And the data pool to draw on is so narrow I’m uncomfortable saying one is immunologically better than the other. But we’ve had some studies (here, here, here, and here), and they’ve shown many similarities and some subtle differences:
Similarities
Both provide a solid first line of defense (i.e., neutralizing antibodies).
Both strengthen a solid second line of defense (i.e., T-cells).
Differences
Negative: Novavax produced significantly lower levels of a specific antibody called IgG.
Positive: Novavax had a more durable response over time (waned less quickly).
Positive: Novavax has fewer side effects, like pain and muscle aches. For this reason alone, I will be getting Novavax this fall.
The new RSV vaccines aren’t covered by insurance?
Medicare Part D covers the RSV vaccine, but some private health insurance plans don’t. In other words, some older adults must pay ~$330 for their RSV vaccine. This is because of two factors:
The adult RSV vaccine is not yet included in the CDC’s annual vaccine schedule, which should be updated in 2024.
CDC’s official recommendation for RSV vaccines is that older adults “may” get the vaccine rather than “should.” Private companies use this language as justification not to cover expenses.
Aren’t there neurological side effects to the adult RSV vaccine?
Clinical trials found a possible safety signal. For more details, see this previous YLE post.
TLDR: Because the number of events was so small, it’s hard to know whether these are coincidental events or reflect a real safety issue. Future data will clarify this, but it will take time for real-world data to accumulate.
When will the maternal RSV vaccine be available?
It will still be a while. While the FDA has officially approved the vaccine, the CDC is not meeting until October 25-27 to determine policy.
Bottom line
A lot is going on this fall. Keep sending us your questions. I hope this helps!
Love, YLE
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH Ph.D.—an epidemiologist, wife, and mom of two little girls. During the day, she is a senior scientific consultant to several organizations. At night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health world so that people will be well-equipped to make evidence-based decisions. This newsletter is free, thanks to the generous support of fellow YLE community members. To support this effort, subscribe below:




Thanks, great list. In the past, FDA restricted Novavax to individuals 18 years and older "for whom mRNA bivalent booster vaccines is not accessible or clinically appropriate" or "who would otherwise not receive a booster vaccine dose."
This language was very confusing to pharmacies, so many of the larger ones (like Costco) "complied" by turning away people seeking vaccines and boosters.
Will there still be restrictions on Novavax this fall? With all the mRNA shots I've received, I'd like to mix it up this time.
I have one employee who still thinks the mRNA shots have microchips and only took the J&J shot. Even after the warning for her age group. This makes the Novavax a welcome option when I urge vaccination in my office!
Frustrating: those who are most needy, such as with autoimmune diseases, can have flares from vaccines. More studies there would be helpful one of these days. IBD, RA, PsA, etc.,. Obviously infection can as well. But vaccine effectiveness in those taking one of the myriad of medications so often advertised would also be welcome. These are the individuals most likely to want and need the vaccine to participate in the world and life.
9/12, noted and thank you!!