A consequential meeting took place at the FDA today. Their external scientific committee—called VRBPAC—met to discuss the national plan for COVID-19 boosters going forward. They essentially needed to answer one question: Do we simplify the COVID-19 vaccine approach in the U.S. and, if so, how?
Here are your cliff notes.
The challenge
No one has a crystal ball for SARS-CoV-2's future, but it will be with us for a long time. So we need to figure out how to mount a proactive, sustainable approach given the rapidly changing landscape.
Some things are helping us right now:
COVID-19 may be finding a more predictable path. (We’ve had Omicron for more than a year.)
A majority of people have been exposed to the virus. Hybrid immunity is robust.
But some things are working against us:
mRNA vaccines are not perfect.
People aren’t getting vaccinated, even if the updated vaccines are working.
~4 million babies per year will be immune naïve.
Different populations have different risks;
COVID-19 is mutating four times faster than the flu.
The U.S. is moving towards privatizing vaccines.
Presentations galore
The amount of information and data packed into this 8-hour meeting was both overwhelming and not very helpful. (See more below.) I won’t go over every detail, but here are some important tidbits.
Who is most at risk?
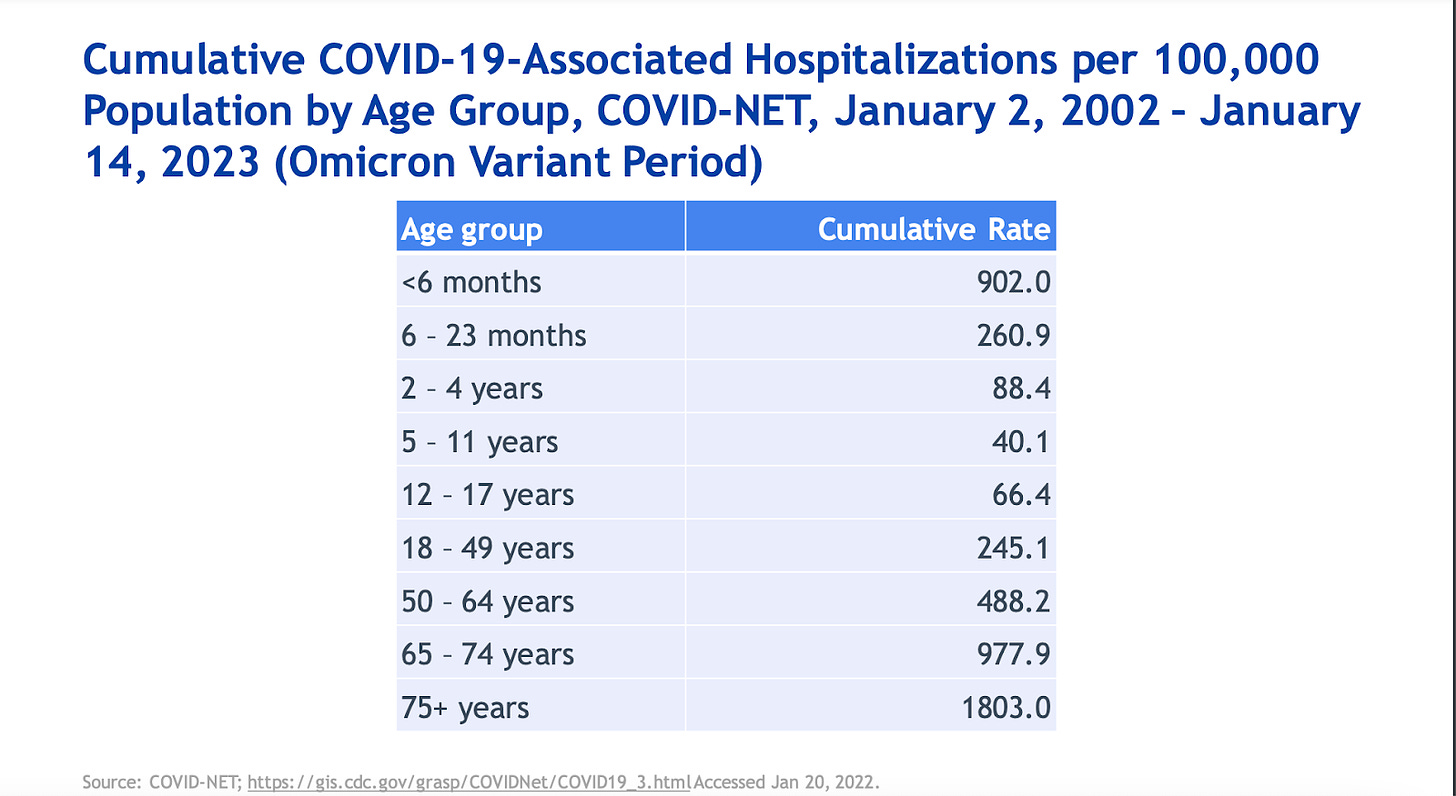
The CDC presented data that is not surprising: most hospitalizations and deaths occur among older adults. Interestingly, children under 6 months are being hospitalized at about the same rates as those aged 50-64 years. This highlights the importance of maternal vaccination during pregnancy.
How well are the bivalent vaccines working against severe disease?
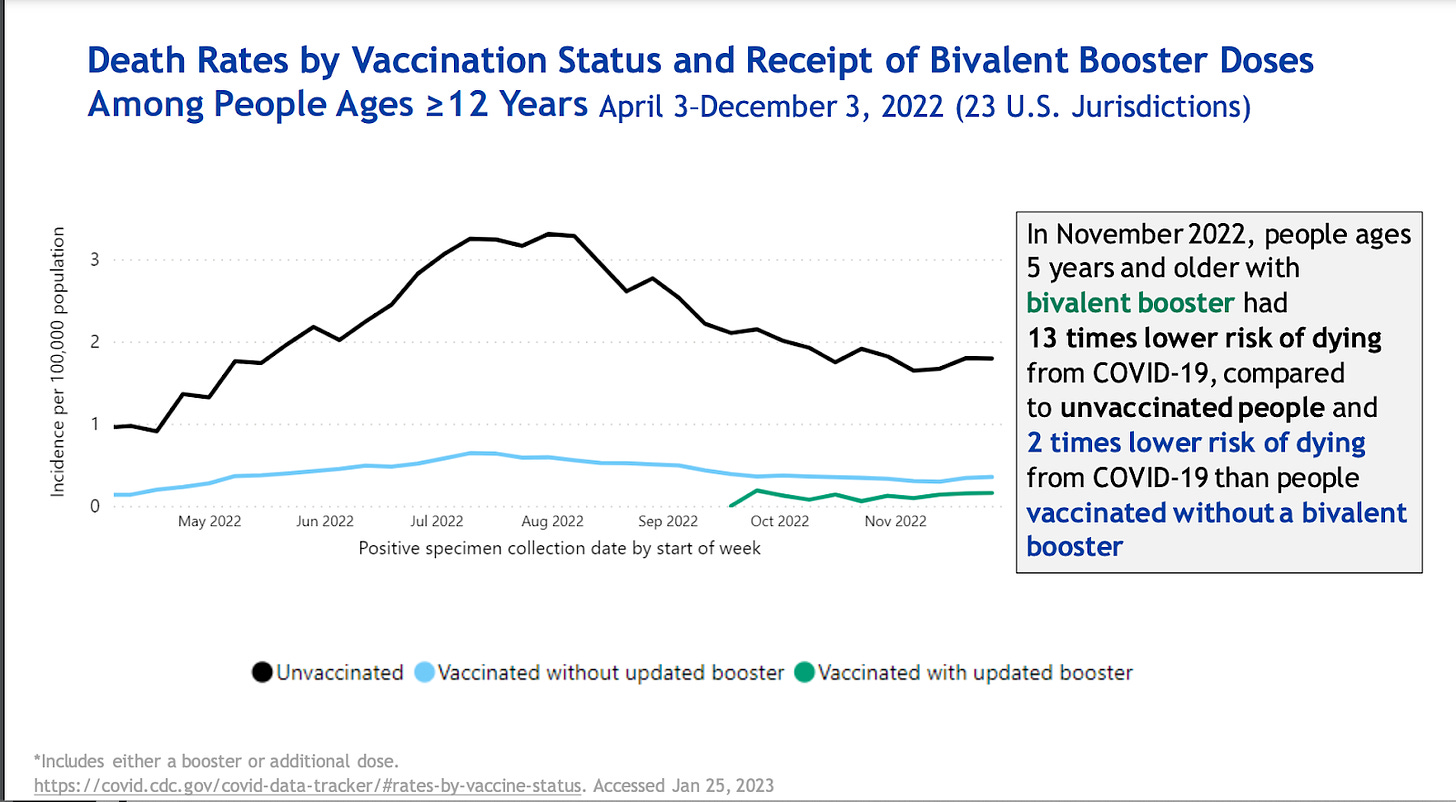
The bivalent vaccines are working well. Adults who received a bivalent booster had 3 times lower risk of hospitalization and 2 times lower risk of dying compared to those who were vaccinated but did not get the bivalent booster. Both were more effective than no vaccination.
There is a problem with this data though: people who got the bivalent vaccine are likely very different, in environment and behaviors (like masking), from people who didn’t get the bivalent vaccine. So, we don’t know if this difference in outcomes results from the vaccine, per se.
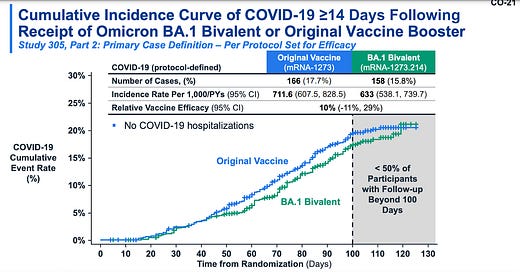
Moderna surprised us today with new data, though: a randomized trial in the U.K. They randomly gave people the original vaccine or the bivalent vaccine (BA.1 formula) as a booster. The bivalent vaccine did better. This really put the debate to rest.
Even though the advantage is small, this data solidified the idea that an updated vaccines “anchors” our response to better respond to the circulating virus. This complements the B-cell data we’ve seen so far, too. (Note: Even Dr. Offit, who’s been critical of the bivalent vaccine, praised the study).
Stroke safety signal?
A highly anticipated analysis on the stroke safety signal after the Pfizer bivalent booster was presented. Some details:
Only one safety monitoring system found a signal. The other U.S. safety monitoring systems did not.
130 people over the age of 65 had a stroke after the Pfizer bivalent vaccine (while 92 events were expected). Among 22 cases investigated thus far (among all 130), three people died. One has been linked to the vaccine.
The safety signal attenuated last week.
Concurrent flu vaccination did not explain this safety signal.
Various countries in Europe, and Israel, have not seen increased risk in their surveillance systems. (This is incredibly reassuring).
Their bottom line message: everyone who is eligible still needs to get vaccinated.
What about Novavax?
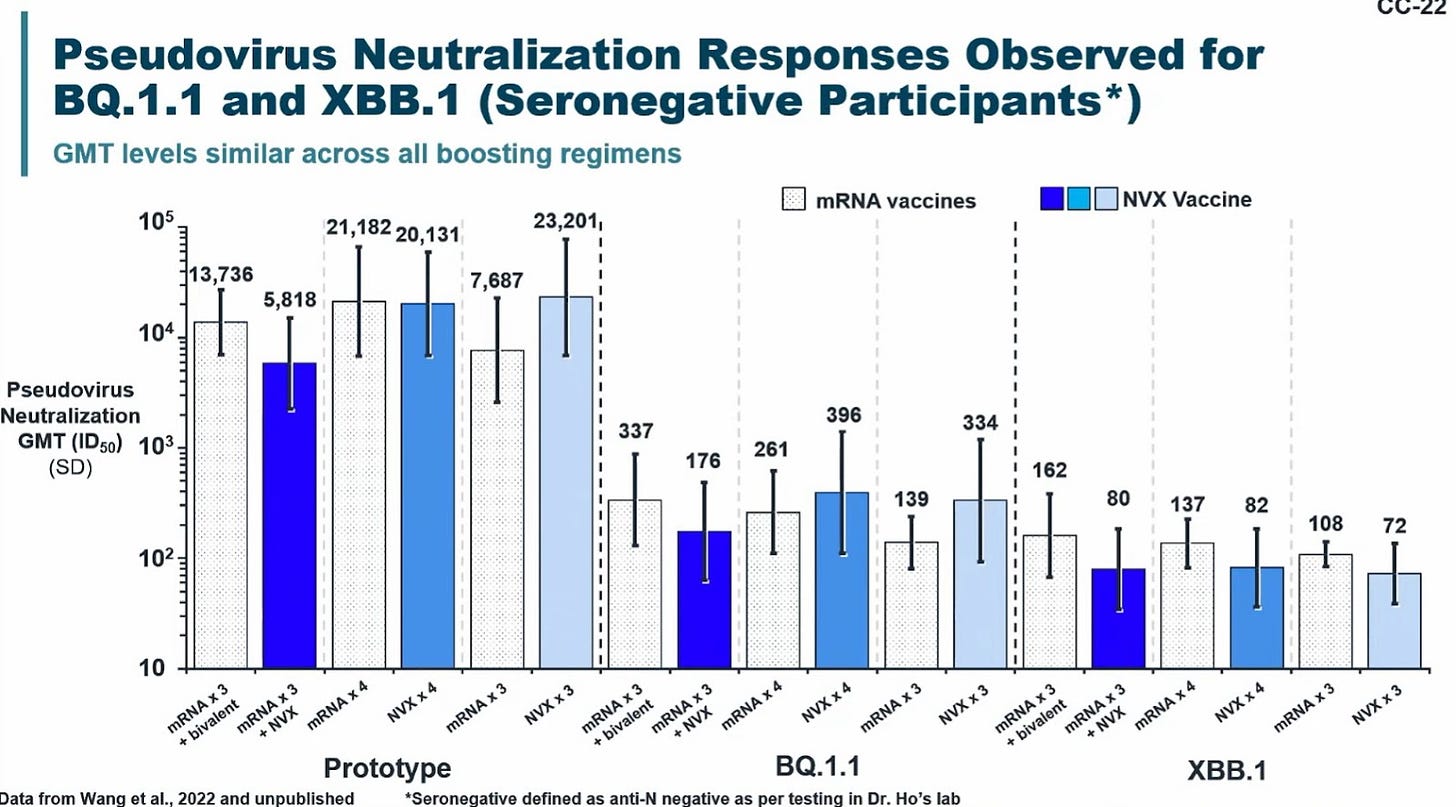
I was excited to see Novavax data. It’s clear this is a solid vaccine.
And their presentation was much more useful than Moderna’s or Pfizer’s. To demonstrate, they included data on mixing Novavax with mRNA vaccines. It looks like no matter how you mix the two, the combinations work about the same way. At least in the short term. I’m very curious to see how this looks in the long term.
The big bummer is they need 6 months to make an updated vaccine. This isn’t ideal given how quickly the virus mutates.
What about next generation vaccines?
There are clearly limitations to the vaccines. We can do better. An NIH presenter said they are focusing on new vaccines that are:
Variant proof;
Longer lasting; and/or
Have enhanced ability to block infection/transmission.
This will take time, though, without an Operation Warp Speed 2.0-level investment or global collaboration. In addition, unlike the first generation mRNA vaccines, we don’t have decades of groundwork. We are really starting from scratch, and it will take time to understand what works and why.
So what is the FDA actually proposing?
They want to replicate the flu vaccine model, but faster:
Flu: Scientists at the WHO meet twice a year. For the Northern Hemisphere, strain decision is in February for a fall vaccine roll-out.
COVID-19: FDA proposes scientists meet in June for strain selection for a annual September vaccine roll-out. This timeline is possible for mRNA vaccines; not for Novavax. So I’m not sure what FDA is going to do about that.
The scientific committee had a lot of questions after this proposal:
Just annually?
The FDA defended a fall only campaign in three ways:
Winter is when the stress on hospital systems will be greatest, thanks to other respiratory viruses.
The FDA thinks it’s already seeing seasonal patterns. (I disagree, although we agree this will eventually happen.)
Once a year eases implementation and decreases confusion for the public, which may help increase vaccine uptake.
Some voting members said it may be annual for now, but not later. Or maybe we won’t even need an annual. We really have no idea.
Nonetheless, it’s clear the FDA thinks the primary objective for vaccines is to do what’s good for the population, which may be different from what’s good for the individual (like getting a booster before a surge).
What about the rest of the world?
The WHO provides universal, annual recommendations for the flu vaccine. However, many are concerned the U.S. is going to dictate what is best because this is where pharma is located. The rest of the world won’t have a choice? It’s not clear how FDA’s plan will be harmonized with the rest of the world.
Why include the original Wuhan strain?
In other words, do we still need to include the original formula or do we just use the updated Omicron formula? One theoretical concern is imprinting. (I’ve written about imprinting before; see more here.) The FDA said this decision will be made at the next meeting.
Elephants in the room that weren’t addressed
I was disappointed this meeting didn’t address practical implications. Maybe this is because VRBPAC is punting policy to ACIP. (VRBPAC/FDA’s primary job is to discuss the effectiveness and safety of vaccines. This is different from ACIP, whose job it is to determine policy.)
Some important unanswered questions:
Who gets an updated vaccine? When? And what dosage? There was an over 1.5 hour presentation on vaccine safety, which mostly focused on the new stroke signal. Myocarditis was brought up, but quickly glossed over. We have yet to see a benefit/risk analysis for bivalent vaccines by age. This is desperately needed for the court of public opinion, where questions remain, particularly for younger folks.
If the vaccine is updated once a year, could people get the “older” vaccine more than once a year? For example, could older adults get a booster before a new surge?
What is the game plan if a random variant of concern comes up? How do we determine when to do this? SARS-CoV-2 is not the flu.
Why isn’t the FDA pushing pharma to do more? A common theme throughout the pandemic FDA meetings is that “it’s too hard to measure anything other than antibodies” and “we don’t have the data needed to make a decision.” The FDA could require sponsors to do detailed investigations, e.g. assessing lymph nodes, bone marrow, and breakthroughs. This would help us understand immunity better, so we can make better recommendations. It’s not clear why they aren’t pushing for this.
Bottom line
It sounds like in the future we will get updated COVID-19 vaccines. Maybe yearly? Maybe a booster for some people (like older adults) but not others? There are still a lot of unanswered questions. If we use the flu model for SARS-CoV-2, it’s a giant leap of faith with no contingency plan. We will need a bunch of luck that it will work. Time will tell.
Love, YLE
A special thanks to my sick toddler stuck at home who “helped” take notes during the VRBPAC meeting:
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, data scientist, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank and is a senior scientific consultant to a number of organizations, including the CDC. At night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support this effort, subscribe below:





I know doctors don’t think this way (I’m married to one), but unless there’s some significant risk associated with getting a second bivalent booster, why not get one six months after the first one for whatever good it might do? I’m one of the rapidly dwindling « never Covid » contingent, and I’d like to stay that way.
It is so frustrating to hear the glacial pace of vaccine development and the lack of political will to do another round of “Warp Speed” for a virus that is killing 3,000 a week and disabling millions.
It is extremely disheartening to hear this inertia from the scientists and scientific bodies whose entire mission it is to be at the cutting edge of science for public health’s sake. If we cannot depend on them during a global pandemic (and it is crystal clear that we cannot), then why do they exist? To minimize and ignore the severe impact this vascular virus is having on entire generations?
I am entirely frustrated.
- signed, a mom in the US South, whose child is immune-compromised, so we have to continue to sit out on life