Today the FDA external advisory committee, called VRBPAC, met to discuss and vote on the safety and effectiveness of two COVID19 vaccines (Moderna and Pfizer) for under 5 year olds. Many of us parents have been waiting a long time for this! Ultimately, the committee unanimously voted to approve both vaccines.
Many parents have fantastic questions. I was in attendance at the FDA meeting; here are the presentation slides. Below I answer questions I’ve frequently received, using data presented at the FDA meeting as well as recent scientific studies.
Does my child actually need the vaccine?
Yes. There is a tremendous burden of disease in this age group. Thankfully the rate of severe disease is lower compared to adults, but this is an inherently flawed comparison because kids don’t die as often as adults. Since the beginning of the pandemic, 442 children aged 0-4 years old have died from COVID-19. If we compare to other vaccine preventable diseases among children, deaths due to COVID19 are highest. We cannot become numb to these deaths.
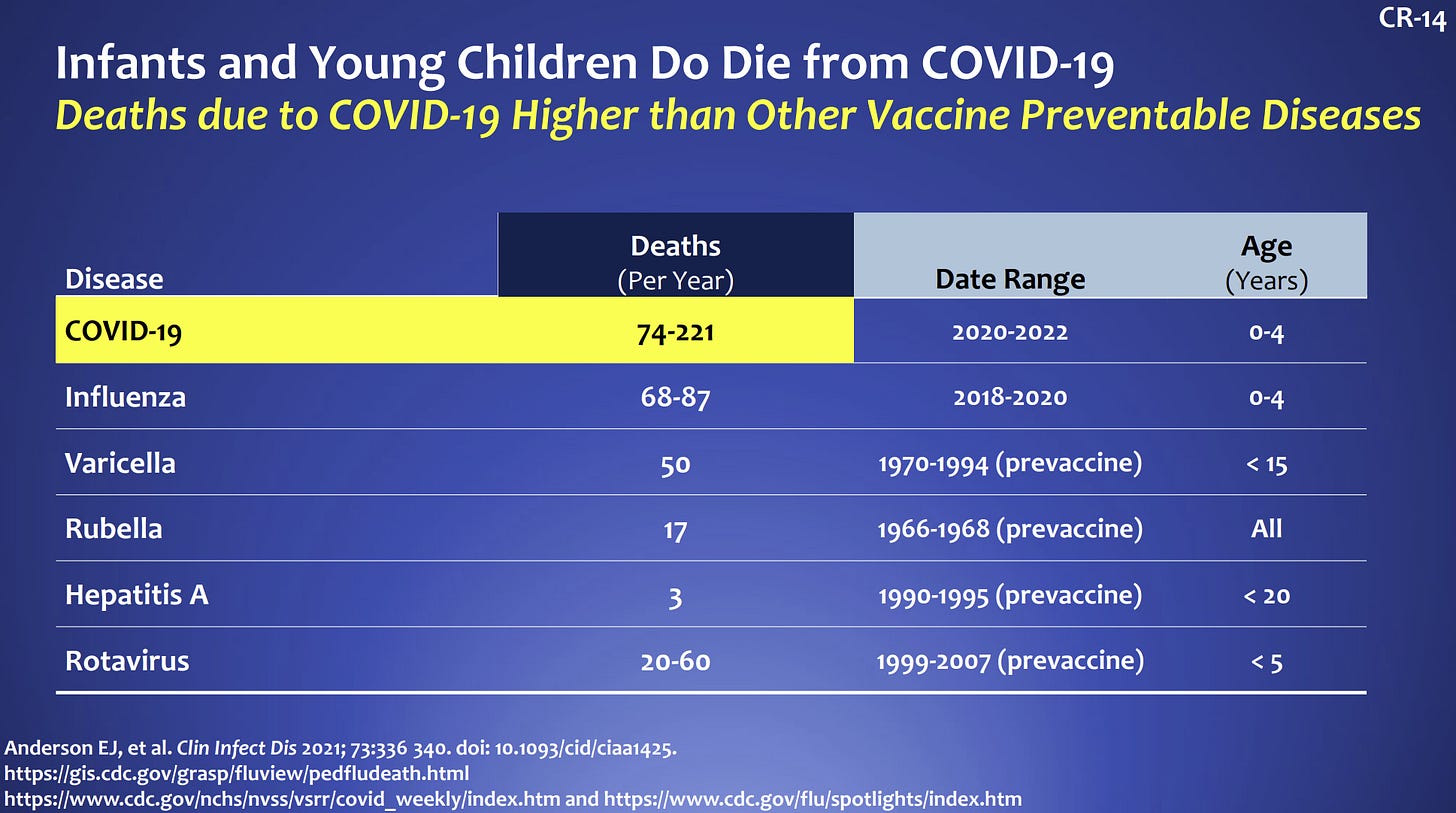
In terms of hospitalization, children, and specifically those under age 5, did not fare well during our first Omicron wave. According to the CDC, children under 5 had the highest rate of hospitalizations compared to other pediatric groups. Among children hospitalized, 1 in 4 ended up in the ICU.

The rate of COVID19 hospitalization was particularly high in October 2021-April 2022 compared to previous flu years and compared to COVID19 hospitalizations the prior year (October 2020-September 2021).

Severe disease is not the only outcome of SARS-CoV-2:
Long COVID19 does occur among kids, and vaccines reduce the burden of long COVID by 15-50%.
We parents know that masking and social distancing very young kids can be nearly impossible. The layers of protection we can employ are less than optimal.
We have frequent, unexpected disruptions in care and schooling of children that contribute to the daily burden of COVID. While not perfect, vaccines will help reduce infections and transmission, inching us closer to less family disruptions.
What if my child was recently infected with COVID19?
This is a great question. We are still trying to understand the durability and breadth of infection-induced protection, especially as Omicron continues to mutate. Very recent scientific evidence shows that 32% of children failed to make antibodies against SARS-CoV-2 after confirmed infection and had mediocre T-cell responses. This isn’t surprising. As I’ve written before, Omicron-induced immunity among unvaccinated people does not protect against other variants of concern. In addition, the quality of response (i.e., memory B-cells and T-cells) is relative to the severity of infection. If a child had a mild infection (which many do), then they likely had a lower viral dose and that secondary protection is less likely. This underscores the importance of vaccinating kids regardless of previous infection.
Vaccines clearly improve protection. I captured the grainy photo below during the FDA presentation today. The blue bar, which represents antibody concentrations after a Moderna shot, significantly increased after vaccination among those who were not infected and those who were previously infected.
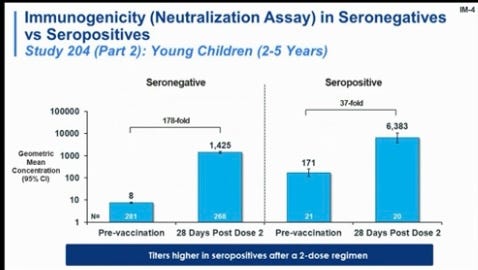
Vaccine + infection is called “hybrid immunity” and more than 20 studies among adults have shown it works fantastically due to complementary and broad protection: vaccine immunity targets the spike protein, and infection-induced immunity targets the whole virus. This doesn’t mean you should purposefully get your child COVID19, but we need to recognize this as a viable path to protection.
What vaccines will be available?
This vaccine rollout is a bit different as, for the first time, two mRNA vaccines will be available at the same time. There are meaningful differences between the two vaccine formulas (see my previous post for details). There were also meaningful differences in the clinical trials. The dosage is different, and more importantly, the number of doses is different. This means the vaccines are not on the same playing field, and we need to be careful comparing side effects and efficacy.
Are the vaccines safe?
Yes. During the clinical trials, side effects were minimal:
For 6-23 month olds, irritability (65% Moderna vs. 44% Pfizer) and drowsiness (40% Moderna vs. 20% Pfizer) were most common.
For 2-5 year olds, pain at injection site were most common (60-70% Moderna vs. 27% Pfizer), followed by fatigue.
For Moderna, 1 in 4 experienced a fever. Side effects were more common after Dose 2. For Pfizer, 1 in 20 experienced a fever, and side effects for Dose 3 were similar to Dose 2. The higher rate of Moderna side effects is due to the higher dosage of RNA.
No myocarditis cases were reported in either clinical trial. This is great but expected news. The clinical trials were not nearly large enough to capture such a rare event. Data from 5-11 year olds show no myocarditis safety signal unlike 12+ year old boys. The leading hypothesis is that myocarditis among teenagers is caused by a combination of increased hormones and genetics. This is a hint that we may not see myocarditis as an issue for our very youngest kids, but data will be closely followed.
In the Moderna clinical trial, there was one case of febrile seizure and rash, which occurred six hours after the first dose. The child went to the emergency department and was released to go home a few hours later. She had a second seizure two weeks later. She stayed in the study and received the second dose without a seizure or fever.
In the Pfizer clinical trial, one potentially life-threatening adverse event occurred in an infant—coffee was spilled on them causing a thermal burn. Pfizer reported it to the FDA. This is an example of the rigor and comprehensiveness behind clinical trials.
Are the vaccines effective?
Yes. The FDA required Moderna and Pfizer to prove immunobridging. This is a process that compares antibodies among this youngest age group to another age group (in this case, 16-25 year olds) in which the efficacy of a vaccine is already established. We already know this vaccine works well; we just need to be sure the dosage works for kids.
Clinical trials found that antibody numbers were comparable to the older age group. In other words, the 2 doses of Moderna and 3 doses of Pfizer worked.
Pfizer and Moderna also reported efficacy against COVID19 disease. Remember that the two aren’t on the same playing field because of different doses:
Moderna reported 37-46% efficacy against disease for 2-5 year olds after 2 doses. And 31-51% for 6-23 month olds after 2 doses.
Moderna has already started testing Dose 3 and will have results this summer. It is clear that this is, at least, a 3-dose series for everyone. A third dose will substantially improve effectiveness for these kiddos.
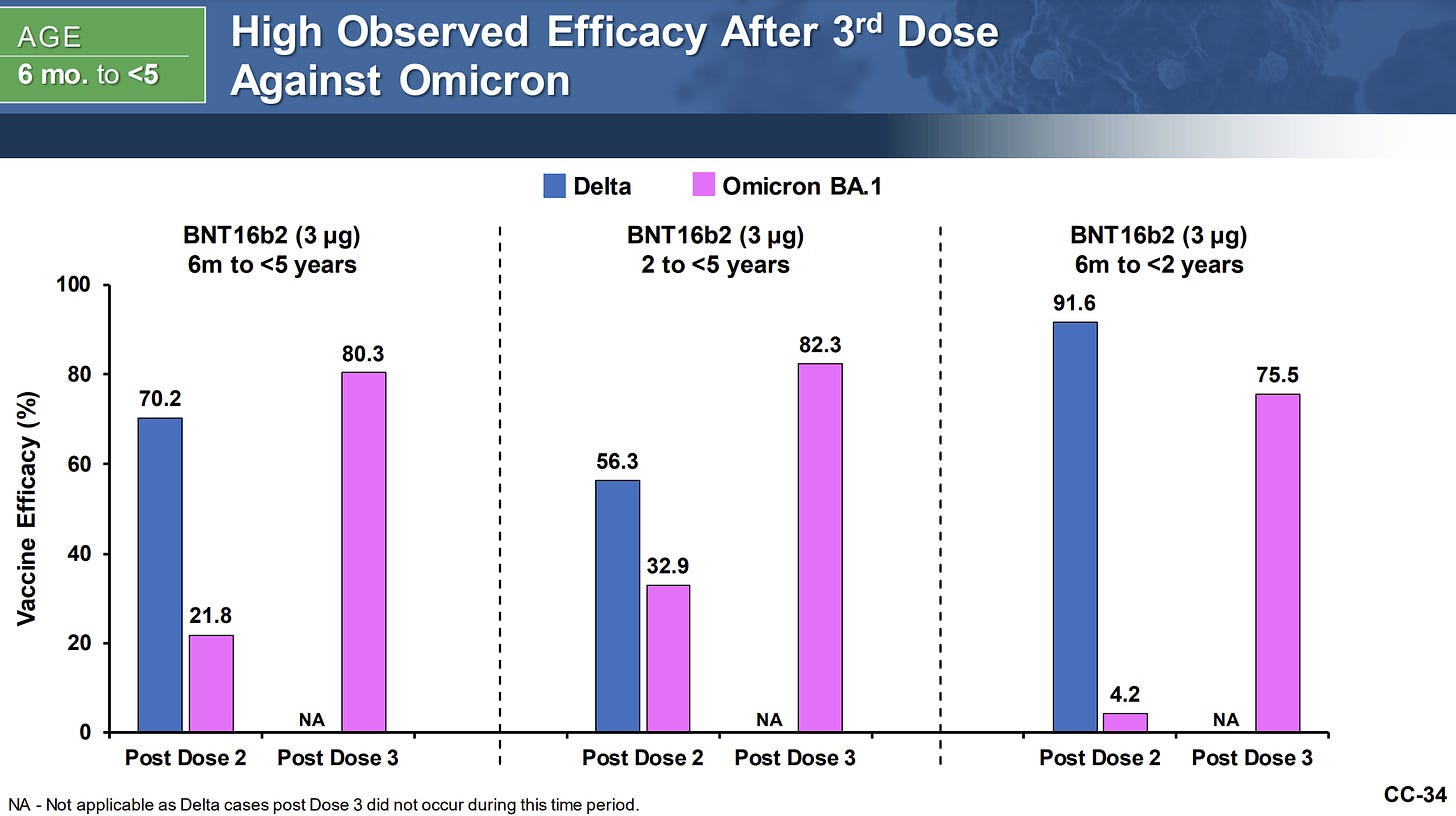
Pfizer reported 80.3% efficacy after 3 doses.
Notice below that after Dose 2, efficacy was close to 0% for the youngest kids. This is incredibly surprising as this means kids are not protected until Dose 3. THIS was the data we were waiting for for such a long time—it explains why Pfizer delayed the 2-dose EUA this past winter. It wouldn’t have passed the test.
We have a hard choice. Which vaccine should my kids get?
You cannot make a wrong decision. Either vaccine is better than nothing, and both help with severe disease and death.
To me, though, the choice is clear. My girls will get Moderna for four reasons:
The confidence in Pfizer’s efficacy is not strong… at all. Efficacy was only based on 3 cases in the vaccine group and 7 cases in the control group. Pfizer didn’t meet the standard protocol of 21 cases. This means the “true” effectiveness is unstable—it could be anywhere between 14% and 81%. We don’t know exactly where. Take 81% with a grain of salt.
Antibodies with Moderna reach the same levels in half the time compared to Pfizer (6 vs. 13 weeks). Kids are not protected until Dose 3 of Pfizer, which is a while.
Moderna confirmed they already started testing Dose 3 (booster) and will have data by this summer. We know that this vaccine is at least a 3-dose series. So, once we get Moderna and Pfizer on the same playing field, efficacy will be comparable. Importantly, Moderna is testing an Omicron-specific booster, not the original vaccine. This is different from the Pfizer trial, in which Dose 3 is the original formula. This is huge. By the end of summer, Moderna kids will likely be on the same playing field as adults, who will likely get a bivalent vaccine this fall.
FDA said they do not know whether the primary series for Pfizer is 3 doses or if this third dose is considered a booster. In other words, it may very well be that Pfizer kids will need a fourth dose for primary efficacy.
I was vaccinated and am currently breastfeeding. Should we delay the vaccine for my baby?
No, do not delay. Antibodies are transferred through breastmilk. In general, breastfeeding is reliably very helpful against GI infections (which will help with COVID), but it’s less clear whether it helps against respiratory infections. B-cells and T-cells are in breast milk too, but their role is complicated. We don’t think B-cells and T-cells in breast milk are helpful in a clinically meaningful way. Get your baby the vaccine.
I was vaccinated while pregnant. Can we delay vaccination to the child?
Vaccination while pregnant is different from protection conferred through breastmilk. Mothers pass antibodies through the placenta that enter the fetus’s circulation. It is clear that this is protective against respiratory viruses once the baby is born. These antibodies do wane quickly, though. (There are optimal periods for vaccination during pregnancy. If you want to maximize antibody transfer, you should get the vaccine during the third trimester.) Just like with breast milk, B-cells and T-cells are transferred to the womb, but their role is complicated, and we don’t think they are helpful in a clinically meaningful way. Get your baby the vaccine.
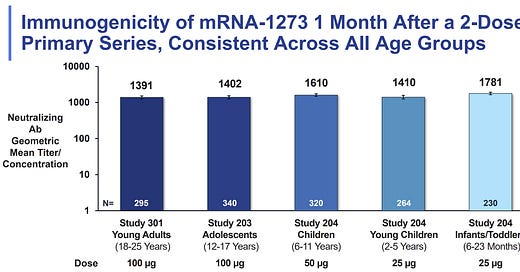
Should I wait for the larger dose if my child is turning 5 soon?
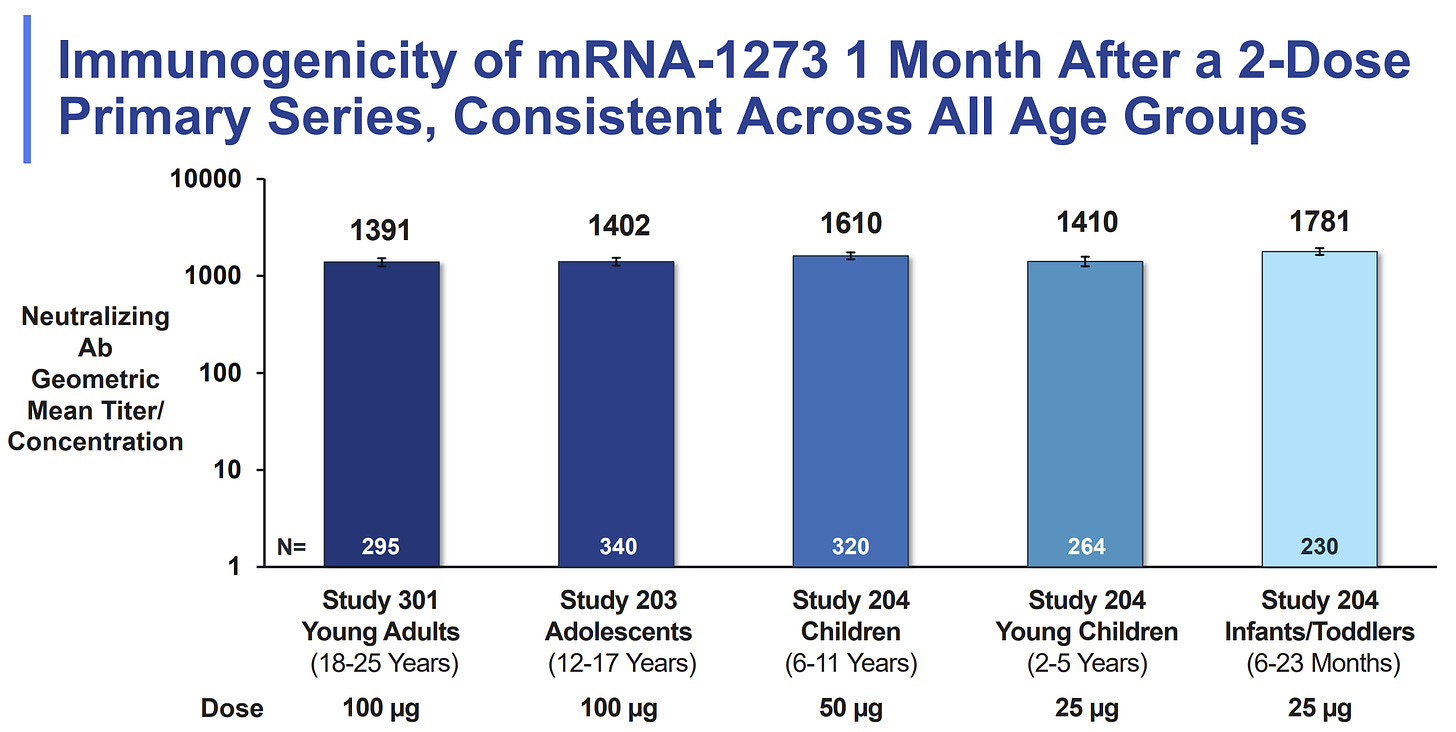
Do not wait. There was a key Moderna slide presented at the FDA meeting today which compared neutralizing antibodies (our first line of defense) across age groups, and thus compared dosages. The high level of neutralizing antibodies for 2-5 year olds (with the smaller dose) were comparable to children aged 6-11 years (with the larger dose). Moderna did test the larger dose (50 mcg) among 2-5 year olds in Phase I of the clinical trial but did not proceed because of high rates of fevers.
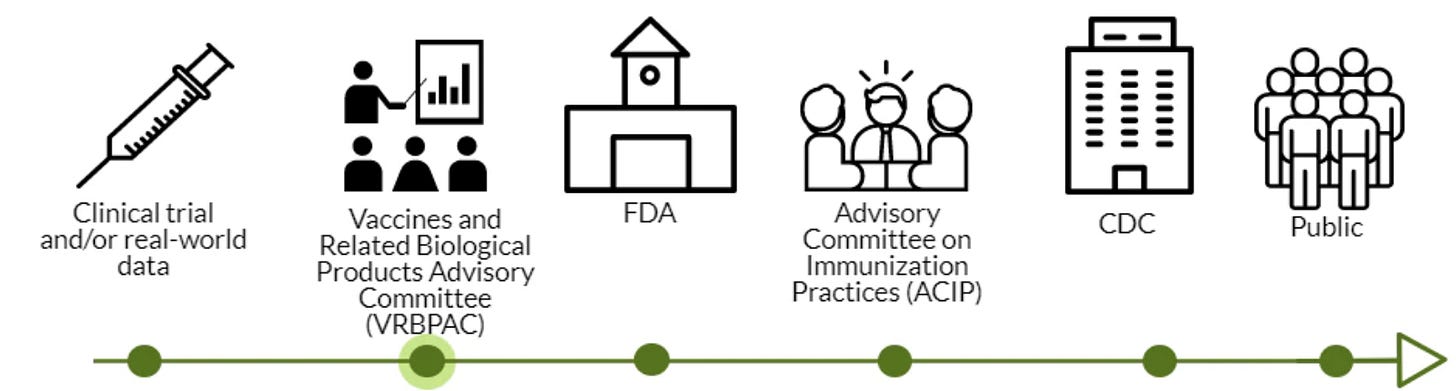
What’s next?
VRBPAC was the second stop in a long process to get the vaccine authorized for emergency use. FDA doesn’t have to listen to their advisory committee, but there’s no reason they would not. The data is clear. This Friday and Saturday the ACIP will meet, and quickly thereafter, the CDC will approve. This means vaccines could be in arms by next week.
Remember to sign up for V-safe!!
When you get your child vaccinated, remember to sign up for V-safe! This is an incredibly important CDC program, which allows us to understand the “real world” safety of vaccines for everyone, including our little ones. Sign up HERE.
Bottom line
This vaccine is safe and effective, and our littlest kids need it. Next week, I will be standing in line to finally get my girls protection from this now vaccine-preventable disease. I hope you will get your kids protected as well, as the evidence is clear.
Love, YLE
I have no conflicts of interest to report with either of these vaccine manufacturers.
In case you missed it:
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank, and at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here:




I have been vaccinated and boosted. I most enjoy the MRNA technology. I read through each of the data sets and graphs - including higher rates of hospitialization. I have not seen a clean statement of reduced risk, more importantly a projection of reduced risk giving children a vaccine designed to protect against an older version of COVID. The antibody activity is fine, we know the key for adults is now the impact on T and B cells of the immune system. Everyone fails to acknowledge the lack of longitudinal data and for low probability events - they will not show in small clinical trials. The vaccines for children may work great - the data available is limited. This should be made clear up front. We are going to give vaccines for old variants of covid - we dont' give children vaccines for old variants of the flu
"...but data will be closely followed".
Having closely followed the roll out here in Australia, I don't feel reassured by your words. Here's a short clip of a very nervous Queensland Chief Health Officer Dr John Gerrard telling us they're "keeping a close eye" on reports of people dying suddenly from myocarditis after a short illness: https://rumble.com/vwhgw9-australian-health-official-vaxed-are-dying-from-myocarditis.html
Note how he corrects himself from "a few" reports to "reports". Months before this press conference, it was reported in our media that two Australian journalists were hospitalised for myocarditis following pfizer (Georgia Clark and Denham Hitchock). Denham Hitchock's case certainly wasn't mild and we don't know if there's permanent scarring that could increase his risk of chronic heart failure in the future.
Here's Queensland Minister of Health and Ambulance Services Yvette D'Ath reporting on a 40% rise in call outs for cardiac arrests, chest pains and respiratory issues:https://rumble.com/v10uifl-queensland-health-minister-cant-explain-sudden-40-heart-attacks-chest-pains.html
A couple of reporters shout out, asking if it's the vaccines but D'Ath dismisses them, saying "Vaccines actually help people stay out of hospital".
Here are testimonials from ICU nurses (general and cardiac) at a Health and Welfare Meeting of the Louisiana House of Reps about the "terrifying" injuries they're seeing from these shots. The nurses all say the injuries are not being reported to VAERS. They speak at the one hour mark: https://house.louisiana.gov/H_Video/VideoArchivePlayer?v=house/2021/nov/1108_21_HW
In this climate of fierce censorship, you'll get a better idea of what's going on by physically going to places yourself or ringing people up and speaking to them directly. You can contact Monte Vista school in CAL; they have 4 cases of myocarditis following the shots in approx 800 students (presumably 50% boys.)
The rate at Monte Vista fits what I've seen in this city of Melbourne, Australia. I don't know that many people but I know two young men (aged 20) who are friends who both got myocarditis after pfizer. They're being seen by the same cardiologist. Neither case is mild. Their prognosis is unknown.
Despite the censorship, you can still find studies that report a higher incidence of myocarditis from the Covid shots. When the study by Patone et al. was redone according to age and gender, investigators found a higher rate of myocarditis in jab recipients skewed to younger males.
As for heart inflammation from the mRNA shots being mild and rare, Ernest Ramirez's son Ernesto aged 16 died after pfizer. Mr. Ramirez didn't know there was any risk of death. He was offered money to keep quiet but refused. There are other cases such as that of Sean Hartman.
Published studies have found permanent damage to hearts following the mRNA shots in the form of fibrosis (Bozkurt et al) and necrosis (Matta et al.), so it's reasonable to think that in some cases extensive damage could lead to death.
You recommend Moderna over Pfizer. We know males of younger age are more at risk of heart inflammation from the mRNA shots and there's a higher risk with Moderna (Sweden, Norway and Finland halted Moderna in under 30s). I therefore don't understand your logic that myocarditis could be less common in the youngest age group, particularly when you're advocating Moderna.
We know the trials in adults were under powered to detect myocarditis but that it still occurred in the real world. There are over 1000 peer-reviewed papers on other adverse effects from the Covid shots, such as transverse myelitis that appeared on FDA slides in December 2020 and is listed in pfizer's postmarketing experience document.
There are websites where you can listen to and read testimonials from people who have been injured by these shots: https://www.realnotrare.com/ https://www.nomoresilence.info/ https://www.jabinjuriesglobal.com/ and a physician led one: https://react19.org/
Even without extensive real world data on injuries, there were major red flags in the original RCTs:
Compare the NEJM report with the FDA one on the Pfizer trial for ages 12-15. Do you know the reasons for the discrepancy in numbers of children who didn't receive a second dose? Do you know the reasons why these children didn't get a second dose?
Brianne Dressen's severe neurological AE was censored from the AZ trial because it occurred after one dose. There's every reason to suspect that could have gone on in the Pfizer trial. Moreover, both Brianne and Stephanie de Garay (whose daughter Maddie was paralysed on the pfizer trial) say trial participants could only report AEs by choosing from a pre-specified list of minor ones.
I question the reliability of any source that makes statements I consider disingenuous. You said Pfizer reporting scalding from coffee (nothing to do with their drug) was an example of rigour. You neglect to mention they passed off a case of paralysis in a child aged twelve as a stomach ache in their published report. It's blatant fraud and criminally negligent not to investigate such a severe case before rolling out to healthy children.
You're strongly urging parents to give their babies and toddlers what the FDA in 2020 considered to be experimental (the EUA document calls the Covid vaccines "investigational") gene therapy (Moderna's SEC filing document from April 2020 states the FDA considered mRNA to be gene therapy.)
If a product is marketed as a vaccine, then certain safety studies don't need to be done; studies in carcinogenicity and genotoxicity were not done because the drug was not "expected' to have an adverse effect in this regard, as you can see on page 29 of Pfizer's document:https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf
Clinical trials to determine safety in reproductive health are ongoing. You may be aware that menstrual irregularities have been reported globally, including in the BMJ. There are two reports of vaginal haemorrhaging in a woman and a child aged 11 on the TGA DAEN website. The child appears on page 35. Note the rat bleeding on the vulva that was euthanized (page 55) in the pfizer study:
https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf
Note also how much of this animal study in safety is redacted. As a parent, does any of this concern you?
The covid inoculations don't reduce transmission at all. The only reason to give these mRNA drugs to children would be to reduce their risk of a severe outcome. Where's the evidence, even in the original clinical trials in adults for the original variant, that they reduce the risk of a severe outcome?
These drugs are, at best, a symptom mitigator with a limited window of efficacy.