On Tuesday night I’m thrilled to join the President of the American Academy of Pediatrics to discuss COVID19 vaccine for young kids. This event is free and hosted by Scary Mommy. This event typically has 20,000 in live attendance, so if you’re interested be sure to register prior HERE. The recording will be available.
Also, if you haven’t completed it yet, please take my Who Are You? survey. The survey will close Sunday. You can find it HERE.
For the past two days, VRBPAC (external scientific advisory committee to the FDA) has been discussing and voting on the following topics:
Moderna Booster: What is the need, effectiveness, and safety of a low-dose (50 μg) booster?
J&J Booster: What is the need, effectiveness, and safety of a J&J booster?
Mixing: What is the effectiveness and safety of mixing vaccines?
These were absolutely fascinating discussions. And proved the utility of having this step in the United States regulatory process. There was a lot of push (from the vaccine sponsors) and a lot of pushback from the review board. Here are cliff notes…
Moderna Booster
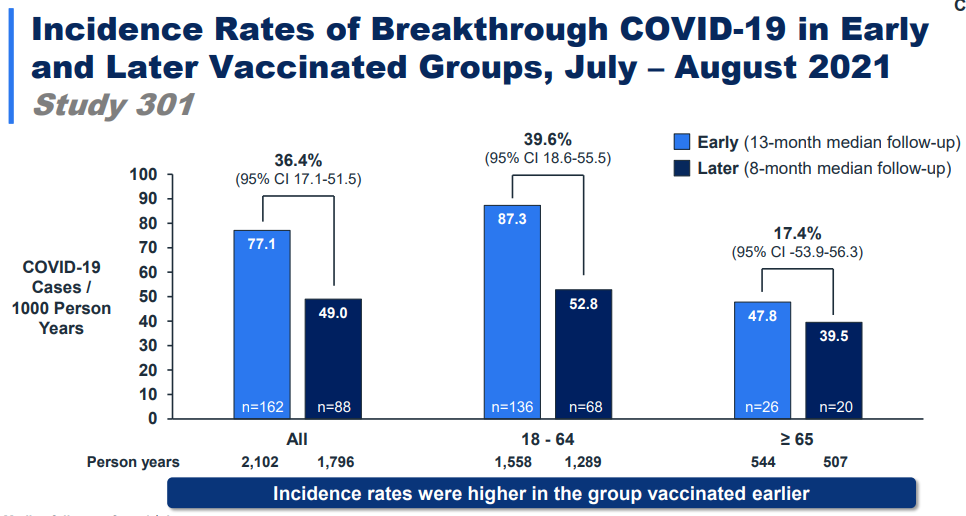
Need: Moderna presented data that people more recently vaccinated had a 36-40% lower rate of “breakthrough” infections compared with those vaccinated longer ago. So, protection is waning and Delta didn’t help.
Effectiveness: Moderna presented data showing that a booster is very effective in rebounding this response, especially among those 65+. The booster worked against the original virus (13-fold increase) and worked even better against Delta (17-fold increase).
The problem is that there is very little data. We had this problem with the Pfizer booster too, but Moderna had even less. Moderna presented their Phase II trial where only 149 adults received the two-dose series and a booster. So, the committee only had data on 149 people to make a decision for 69 million people. The FDA said this was enough data, but the committee members voiced uneasiness. For example, Dr. Patrick Moore said:
“I've got real issues with this vote ... [it's] more of a gut feeling rather than really truly serious data. I think it's very important that companies really take seriously that we need to see good, solid data, and it needs to be explained well.”
Dose: There was significant discussion about the dosage. Moderna applied for a low-dose booster (50μg, which is half the dosage of the original series). During this meeting, they indirectly cited three reasons for the lower-dosage:
Should be enough protection for a robust response;
Fewer reactions, such as fever and muscle aches, compared to the higher dosage;
Global pressure. By dividing the vaccines in half, there will be more vaccine available for global supply.
Several advisers weren’t convinced, though. Some voting members said a low-dose booster could cost people the potential benefit of a full strength booster over time. It may have tremendous impact on durability.
There was also confusion on what happens with immunocompromised now. They already got the full 100 μg booster. So do they need the 50 μg now? This will hopefully be cleared up by ACIP next week.
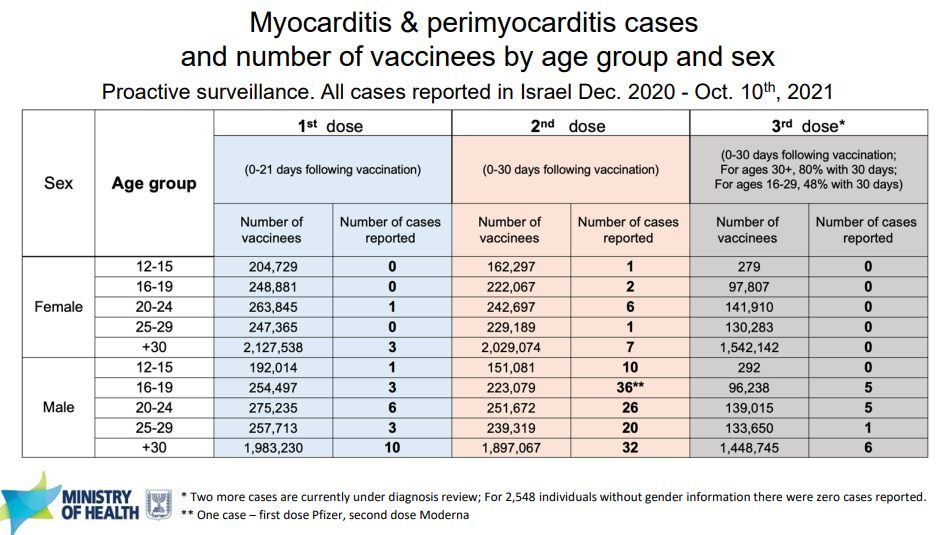
Safety data was based on 171 people, so data was scarce and certainly wasn’t large enough to find rare safety signals, like myocarditis. Thankfully, during this meeting, Israel was willing to come back to the FDA meeting and present their updated data on the safety and effectiveness of their universal booster program. It’s important to note that they are only using Pfizer doses, but the myocarditis data is still applicable.
Thus far, Israel has boosted 3.7 million people and it’s not any risker than the original series. In fact, in terms of myocarditis, a booster is less risky because of the longer period between doses. Israel reported only 17 cases of myocarditis/pericarditis after the booster. All these cases were among males.
Decision: Ultimately, VRBPAC unanimously voted (19-0) to recommend the Moderna booster for three specific groups (which are the same exact groups for Pfizer):
65+ year olds
18-64 year olds at high risk of severe COVID19
18-64 year olds with high exposure to COVID19
J&J booster
Need: J&J approached this very differently than the mRNA sponsors. J&J asked to authorize a booster not because of waning immunity but as an opportunity to increase efficacy against variants of concern. J&J even went one step further and said it is more durable than mRNA vaccines. The slide below was presented, which showed that Pfizer and Moderna had a 34-44 fold decrease after 8 months, compared to J&J which has no change. It’s important to note that this is based on data from 8 people. It has been published in New England Journal of Medicine (as of this morning).
There was a lot of skeptical discussion, though. There has been a number of real-world studies showing waning effectiveness and suboptimal efficacy from the J&J vaccine. The FDA even went to the mic and said J&J ignored real-world data in their presentation. The FDA pulled up this study that was peer reviewed and published in NEJM which showed a much lower efficacy compared to the mRNA vaccine. The CDC came to the mic too and summarized another study showing the same thing. After this discussion, it was clear: Without a booster, the J&J vaccine did not protect people well.
Effectiveness: Nonetheless, J&J presented data from their clinical trial of >9,000 people. A booster significantly increased protection across all variants of concern.
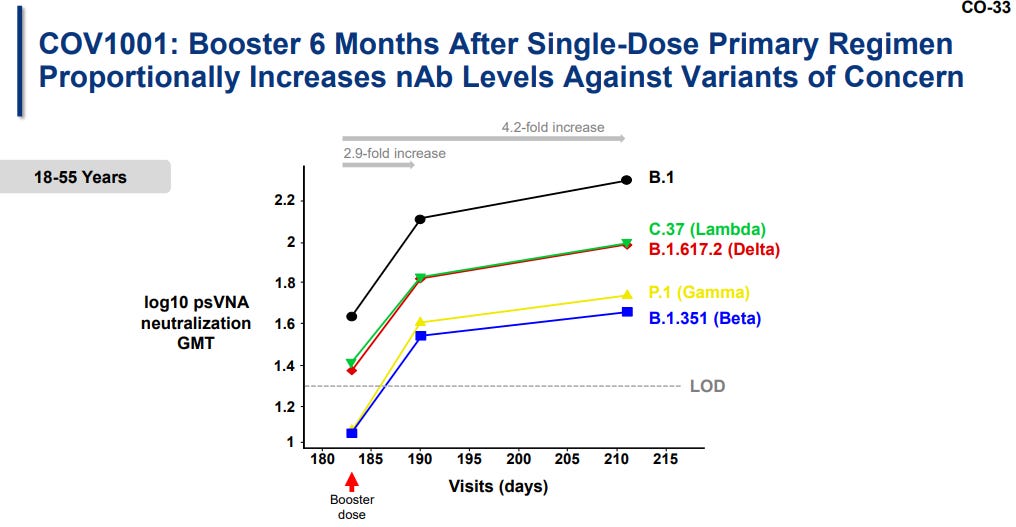
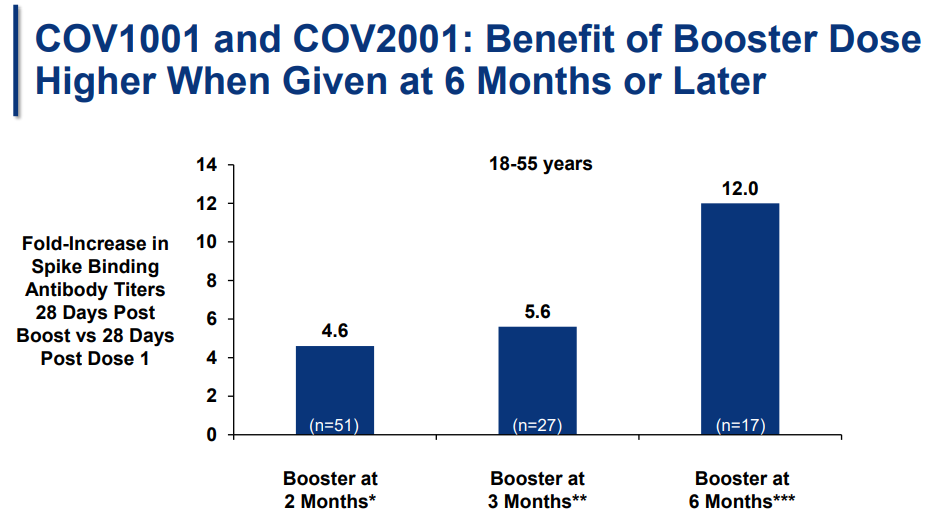
Timing: When should people get the booster? In a smaller study, J&J studied the effectiveness of giving a booster at 2, 3, and 6 months after the original vaccine. J&J advocated for a 6 month booster by showing protection was highest then (see slide below).
But this study was very small. VRBPAC members voiced concern that they were making a decision for millions of people based on data from 17 people aged 18-55 years old. If we make this a 2-month booster, then this should really be considered a two-dose vaccine NOT a booster. J&J does not want this to be a 2 dose vaccine (probably because there’s a marketing advantage for some parts of the world for a single dose vaccine).
Safety: J&J reviewed the safety of a booster. Overall, benefits continue to outweigh the risks. But there are rare side effects:
Blood clots: After EUA, 133 cases of thrombosis with thrombocytopenia (special type of blood clot) were identified; only 73 cases met criteria for investigation. This equates to 2.1 cases of blood clots per million doses. Unfortunately, 12 people died. Data showed a booster is more safe in regards to blood clots compared to the primary shot.
Guillain-Barre: After EUA, 162 cases of Guillain-Barre Syndrome (a progressive but generally reversible paralysis) were identified. This equated to 7.5 cases per million doses. There was 1 fatal outcome. This rate was higher than the background rate (1-5 cases per million).
Decision: Ultimately, VRBPAC voted in favor (19-0) to recommending the J&J booster for anyone 18+ at least 2 months after the single dose primary vaccination.
Mixing
VRBPAC also discussed mixing vaccines. But before they got to this presentation, J&J made it clear this morning that they didn’t support this policy. J&J said there are “questions that remain about how safe heterologous boosting is and how durable the response to a heterologous boost will be.” J&J wants the J&J shot boosted with J&J. (Remember there may be conflict of interest here).
Regardless, VRBPAC reviewed data from a mixing study that was released this week. I talked about this study in great detail here. It’s very compelling data and clearly safe and effective.
VRBPAC didn’t vote today because the FDA didn’t have time to assess the legitimacy of this data. Instead, the FDA wanted to hear the advisory committee’s opinion on potentially approving mixing vaccines.
Overall, VRBPAC was impressed with the mix and match study. But there were differing thoughts about how to move forward:
Some said that it’s hard to know the impact of mixing because we don’t know if neutralizing antibodies correlate with protection. Also, if we go forward does this really have to go through the FDA if we expect full licensure in months to come? Some said we can just wait a few months to start mixing.
Others urged the FDA for a quick EUA, so people can have the benefit of mixing as quickly as possible. This will be important for vaccine access, it will provide flexibility, and it will aid in vaccine mandates. The CDC also made it clear that they need allowable language from FDA to make a policy recommendation.
Next Steps
Assuming that the FDA agrees with it’s advisory committee, ACIP and CDC will discuss policy decisions next week on all of the above. The meeting is scheduled on October 21 and the agenda is here. If the CDC and FDA ultimately align, then it’s legal for some groups to get the Moderna and J&J vaccine booster.
Have a great weekend.
Love, YLE





What’s your opinion regarding the simultaneous administration ( in different anatomical sites ) of COVID and Influenza shots ? Thanks.
https://www.lji.org/news-events/news/post/lungs-of-covid-19-survivors-teem-with-virus-fighting-memory-cells/