By now you’ve seen that Johnson and Johnson (JJ) has been stopped in the United States. My morning has been inundated with emergency meetings. While I catch my breath, I can give you a quick run down of questions I’ve been answering…
What happened?
Among the 6.8 million doses of JJ have been administered thus far, there have been 6 reported cases of a “rare and severe” blood clot.
All six cases occurred among women between the ages of 18 and 48
Symptoms occurred 6 to 13 days after vaccination
Of these 6 women, 1 has died and 1 is currently hospitalized
The CDC and FDA have recommended a pause in the use of JJ vaccinations until we can investigate these cases more. This “cluster” of cases indicates a possible safety signal.
Is this just due to an abundance of caution?
Yes. The CDC and FDA are being overly cautious, but rightfully so. Blood clots are rare but serious. It’s important that we make sure this is not a statistical anomaly but is a true safety signal, especially given what has happened with AstraZeneca (see below). It’s worth pausing and investigating and planning for future distribution.
How is it possible that this kind of thing wasn't caught for JJ?
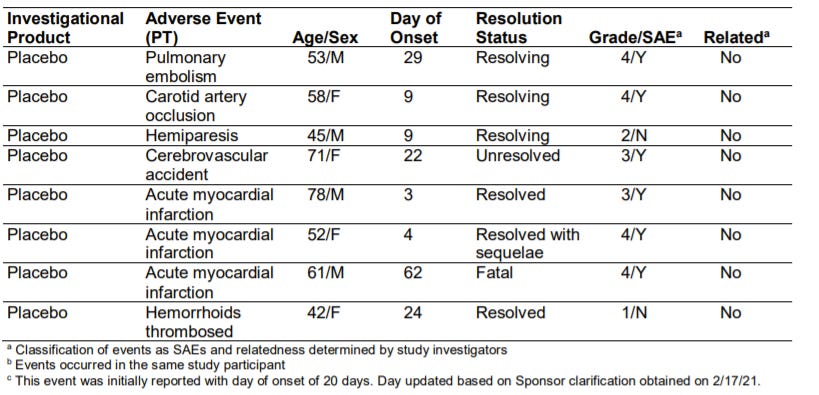
This was caught in the JJ Phase III clinical trial. There were slight imbalances for “embolic and thrombotic events”, which the scientists said may be due to the vaccine. For example, there were 15 events for the vaccine but 10 events for the placebo. Because this event was SO rare, we needed more data to understand whether this safety signal was true or not. The scientists even said (page 7 in this document): “Data at this time are insufficient to determine a causal relationship between these events and the vaccine.”
Here is a table of those events in the clinical trial (see page 46-47 here). And here’s a previous post of mine that talked about this data.
From this list, there were 5 specific events that the “contributory effect of the vaccine could not be excluded based on FDA assessment of the clinical information provided”:
A 25-year-old male with no past medical history and no concurrent medications experienced a transverse sinus thrombosis on Day 21 following vaccination
A 30-year-old female with hypothyroidism, obesity (body mass index: 36.5 kg/m2), headaches, anxiety and depression and use of multiple medications including medroxyprogesterone, experienced a pulmonary embolism on Day 3 following vaccination.
A 52-year-old male with obesity (body mass index: 32.4 kg/m2) experienced a deep vein thrombosis (DVT) on Day 27 following vaccination.
A 63-year-old male with type 2 diabetes, hypertension and osteoarthritis experienced a DVT on Day 23 following vaccination
A 49-year-old female with no past medical history and medication use including medroxyprogesterone experienced hemiparesis on Day 28 following vaccination.
What tells us they won't find something like this with Pfizer and Moderna?
These safety signals did not show up in Pfizer and Moderna clinical trials and did not show up thus far in the “real world”. In the United States, 97 million doses of Pfizer and 84 million doses of Moderna are in arms today.
It’s also important to note that this blood clot connection was made for AstraZeneca (AZ; see here and here for more details). This is important because AZ is the same “type” of vaccine biotechnology (called a viral vector or adenovirus vaccine). JJ and AZ BOTH showing these safety signals (and Pfizer and Moderna not showing these signals) isn’t necessarily a coincidence.
The Sputnik V (Russian vaccine) is also this type of vaccine. This vaccine is not being distributed in the U.S., but it might be time to look closely at these adverse events, on a global level, too.
Wasn’t the AstraZeneca (AZ) paused elsewhere and later restarted? What can we learn about this regarding JJ in the U.S.?
This is correct. After an intense investigation, the European Medicines Agency review board concluded that the benefits of the AZ vaccine far outweighs the risks. So, each country who rolled out AZ is doing something different now regarding distribution:
Some are not allowing AZ for younger populations because these events are only popping up for younger populations (aged 55 less; I think this makes the most sense).
Other countries aren’t allowing AZ at all
Other countries are allowing AZ for everyone
Regardless of what the country decided, it’s clear that this has had detrimental impact on vaccine uptake in Europe. We (epidemiologists) are very worried about this JJ news and what it will do regarding vaccine uptake overall (JJ or not).
What now?
Scientists will jointly examine possible links between the vaccine and blood clotting events and determine whether the FDA should continue to authorize use of the vaccine for all adults or limit the authorization. CDC’s external advisory board (called ACIP) has an emergency meeting tomorrow at 1:30pm CST regarding this subject. I will be in attendance and happy to provide cliff notes.
Alright, that’s it for now. What other questions do you have?
Love, YLE





Hi Dr Jetelina- can you tell me how this compares to the risk of blood clots from oral contraceptives? A risk that our physicians are generally okay with us taking?
Random occurrence of venous thrombosis is annual rate of 1 in 1000 for adults (Cushman, 2007; CDC, 2020). So given we are talking about 1 in over 1 million (vaccinated with J&J vaccine) the fact they stopped the usage of the vaccine with no attributable causal effects is ridiculous.
Are we now replacing science with irrational fear when making decisions?
Sources:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2020806/#:~:text=Venous%20thrombosis%2C%20including%20deep%20vein,than%20women%20in%20older%20age.
https://www.cdc.gov/ncbddd/dvt/data.html