Only two days until the FDA meeting (Feb 26) where an external advisory group will meet to discuss (and ultimately recommend or not recommend) the Johnson and Johnson (J&J) vaccine for emergency use in the U.S. This meeting is important for a couple reasons:
This is the first COVID19 vaccine up that is not an mRNA vaccine (I’ve talked about the J&J vaccine HERE and the biotechnology HERE)
This is the first vaccine with one dose
In preparation for the meeting, this morning the FDA released a 62-page document and the sponsor (J&J) released a 118-page document with trial details and results. This is the first time we (the public) have seen the clinical trial data. Yes, I read it all. Here are your cliff notes:
Number of Participants
-A total of 43,783 people had 2 months of follow-up data and were included in this report (21,895 in the vaccine group and 21,888 in the placebo group)
-Only adults aged 18+ years were included (this is different than Pfizer)
-People with comorbidities were included (40.8% of the sample)
-60+ years with comorbidities were included (28% of the sample)
Efficacy
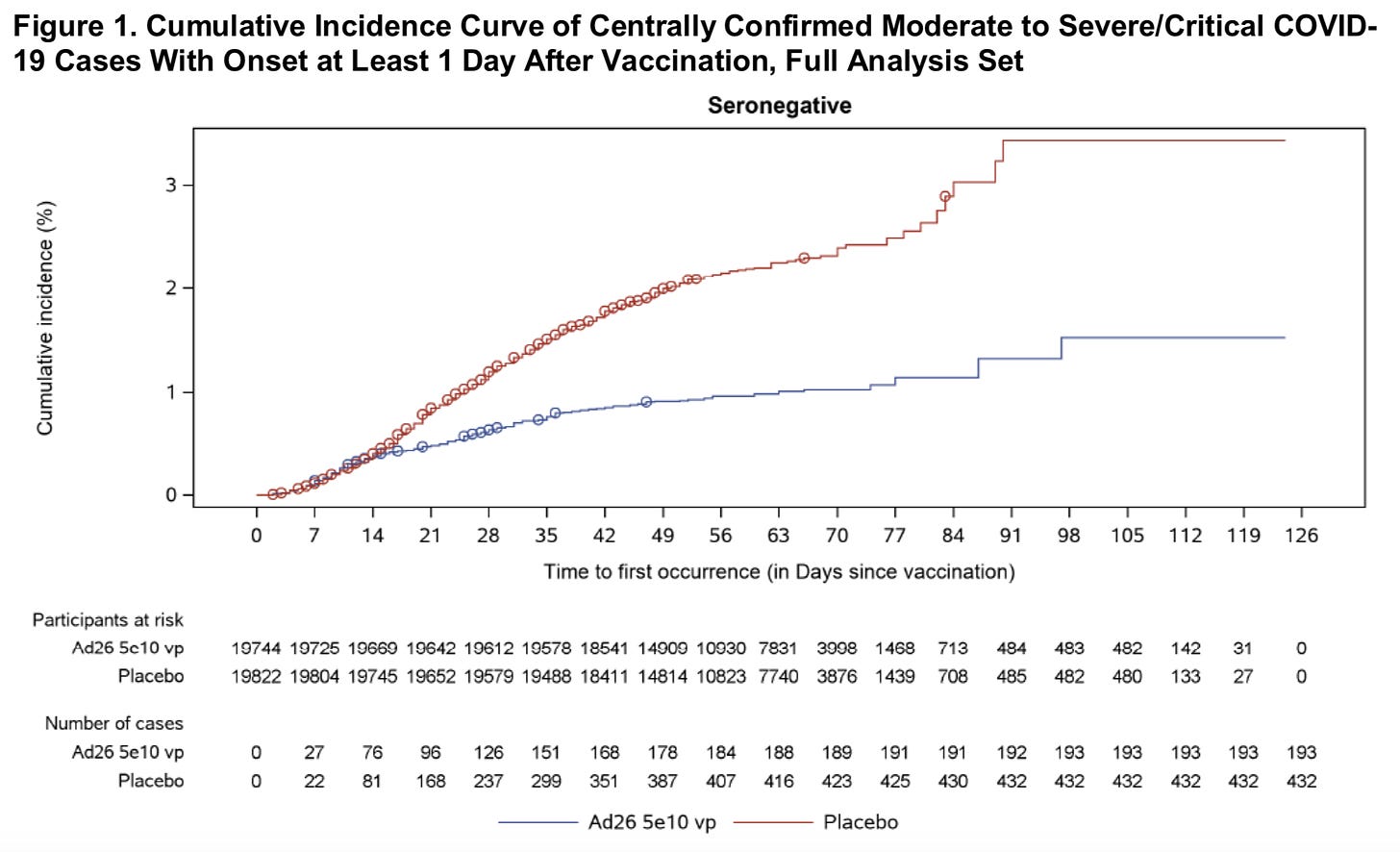
–66.9% after 14 days of vaccination; 66.1% after 28 days
-Efficacy didn’t meaningfully differ by age, sex, race, ethnicity, or comorbidity
-Efficacy did differ by region, but still looks good: 72% US; 64% S. Africa; 61% Latin America (this is likely due to the variants)
Severe Disease/Death
-COVID19 cases needing medical intervention (like ventilation or ICU admission): 0 in vaccine group; 5 in placebo group
-COVID-19-related deaths: 0 in the vaccine group; 7 in the placebo group
Asymptomatic Disease (this is big)
-From Day 1 through Day 28 (after vaccination), the vaccine doesn’t have an impact against asymptomatic infection
-However, after Day 29, 74% efficacy against asymptomatic infection
Safety
-Adverse reactions were reported at higher rates in vaccine group (55.1%) compared to the placebo group (35.1%) and at higher rates among younger (compared to older) participants.
-Most frequent reactions were: headache (vaccine vs. placebo: 38.9% vs. 23.7%); fatigue (38.2% vs. 21.5%); muscle aches (33.2% vs. 12.7%), nausea (14.2% vs. 9.7%); and fever (9.0% vs. 0.6%). These rates are a bit lower compared to the Pfizer and Moderna rates
-No reports of anaphylaxis
-There were slight imbalances for the following rare severe events, which may be due to the vaccine
“Urticaria” (hives): 5 vaccine vs. 1 placebo
“Embolic and thrombotic events”: 15 events vaccine vs. 10 events placebo
“Tinnitus” (ringing of ears): 6 vaccine vs. 0 placebo
-Bell’s Palsy was balanced: 2 vaccine vs. 2 placebo
-25 deaths were reported in the study (5 vaccine, 20 placebo), which represent a rate that occurs in the general population. 7 deaths in the placebo group were due to COVID-19 infection (none in the vaccine group)
Pregnancy Substudy (DART)
-J&J included results for their DART pregnancy study, where scientists gave rats the vaccine
-Rats did not have any adverse effects on female reproduction, fetal/embryonal development, or postnatal developmental. We are not surprised, but still good to know.
Bottom Line: Things are looking good. Real good. This is a solid study, the vaccine works against severe disease and the new variants, AND the vaccine will be easily disseminated to the masses (it’s only one dose and has easy storage). I would be very surprised if this was not authorized for emergency use. This morning we also learned that asymptomatic disease (a good indicator of transmission) is reduced with vaccination (whoo hoo!).
Friday’s FDA meeting information HERE. Like always, I’ll provide you with the meeting cliff notes for this too.
Love, YLE




Pre-vaccine, there was a lot of information on COVID long-haulers, people who sometimes had very mild to moderate symptoms, but whose symptoms persisted, causing long-term impacts. Do we have any information on people who received a vaccine, and only had mild to moderate symptoms, if there are “long-haulers” in this group as well?