Today ACIP— the external advisory committee to the CDC— met to discuss the bivalent fall boosters in the United States. This committee’s main purpose is to evaluate the safety and effectiveness of vaccines as well as policy recommendations for dissemination (who should get one and when).
This was a much anticipated meeting, as we have seen barely any data on the BA.4/5 bivalent booster. We also had some other burning questions. Here was the agenda for the meeting. Here were the presentations. And here are your cliff notes.
(If you haven’t already, be sure to read my previous post first. I’m picking up from there.)
Safety
Moderna and Pfizer presented data on the safety of the BA.1 bivalent vaccine among humans. We have data on more than 1,400 adults. While this is not the vaccine being rolled out in the U.S. (we are rolling out the BA.5 bivalent vaccine instead), the differences between BA.1 and BA.5 boosters are minimal. Literally the difference of a few amino acids—like a few letter edits on a Word document. We aren’t changing the number of words in the paper (like dosage of RNA), or the content of the paper, or the platform (like Word to Excel). Because of the minimal change, we are confident that BA.1 bivalent safety data will accurately reflect BA.5 safety.
In general, the rate of side effects from the BA.1 bivalent booster were the same or lower than from the original booster formula or the original series. For example, Moderna found 4% of participants had a fever after the bivalent vaccine (dose 4) compared to 16% who got a fever after the original series (after dose 2). There were two severe adverse events after the bivalent vaccine (prostate cancer and bone fracture), but they were not linked to the vaccine.

Effectiveness
We don’t know the effectiveness of the BA.5 fall booster among humans. Given the rapidly evolving virus, we need to triangulate four other data sources:
Bivalent BA.1 among humans. Compared to the original formula, the number of neutralizing antibodies (i.e. our first line of defense) induced by the bivalent BA.1 vaccine was better—about 1.22 times better. This isn’t amazing, but it isn’t terrible. This means that we will likely see a slight increase in vaccine effectiveness against infection, but not a huge jump.
Bivalent vs. monovalent. Many wonder why we need a bivalent vaccine instead of monovalent (with only the Omicron formula). Pfizer showed us why. If Omicron continues to mutate, we would be okay with monovalent. However, if we get another random variant, say Delta again, the monovalent wouldn’t be as protective as the bivalent. Bivalent is key in providing broader protection taking into account the unpredictability of the future.
Bivalent Beta vaccine. We hope that an updated bivalent vaccine will last longer compared to the original vaccine. But we don’t have much data. We have the duration that the bivalent Beta boosters lasted. Moderna showed that bivalent Beta remained durable at 6 months. If this translates to our BA.5 vaccines, this would be great news.
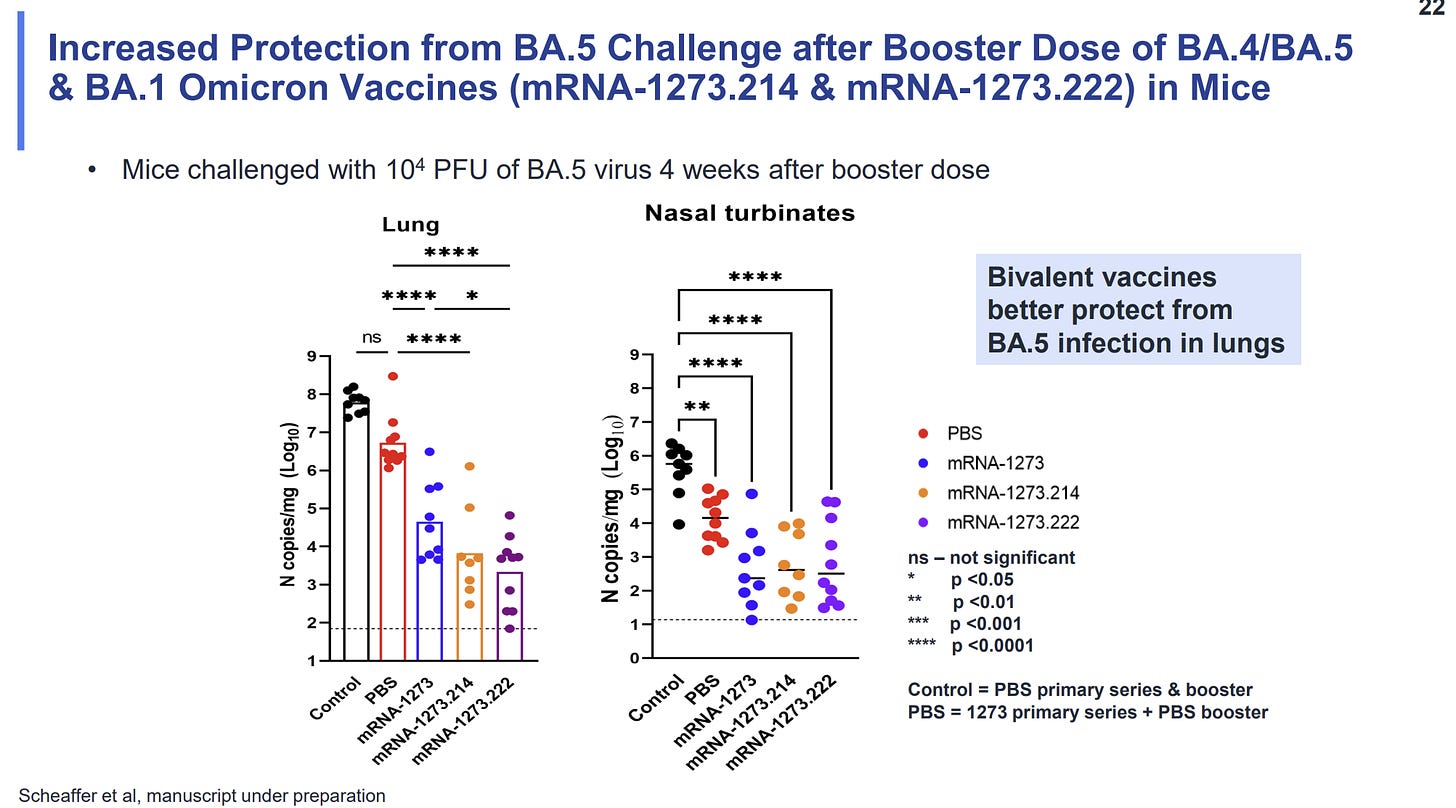
BA.4/5 mice data. The only data we have on the bivalent BA.5 fall boosters is from mice. Honestly, there wasn’t much data presented today, but what we did see was promising. Moderna highlighted that the bivalent BA.5 vaccine is particularly better at reducing replicating virus in the lungs than other vaccine variants. This means that the BA.5 bivalent may be similar or even slightly better than the BA.1 bivalent vaccine.
Benefits and Risks
CDC followed the vaccine manufacturers’ presentation with their fantastic benefit/harm analysis, as per usual.
Benefits
Reduce disease. Models show hospitalizations and deaths will increase this winter. How big this wave will be is dependent on viral mutations, booster uptake, behaviors, and a lot of other things. But if we have a booster campaign that matches the influenza vaccination rate, we have the potential to avert 101,000 deaths, 1,028,000 hospitalizations, and 24.9 million infections. That’s a lot. And equates to diverting $62 billion in medical costs.
High interest. I was shocked to see that 72% of Americans in a survey said they will definitely or probably get the updated Omicron vaccine. If this is truly the case, this fall booster will help… a lot.
Limited imprinting. I need to update my last original antigenic sin post, but we’ve gotten some great news suggesting an improved response after the bivalent vaccine. This suggests bivalent vaccines expand immune response, which is great news.
Risks.
Myocarditis. We are still seeing a rare, but real, safety signal of myocarditis after a booster among male adolescents and young males. But keep in mind the risk is smaller than after dose 2 (thanks to the longer time period), and the risk of myocarditis after COVID19 infection (compared to vaccination) is 1.8 - 5.6 times higher among young males.
Discussion and vote
Some voting members voiced uneasiness around the lack of human data for BA.5 boosters, but we rely on similar data and background with flu every year. Ultimately, ACIP voted 13-1 for boosters this fall:
Pfizer: Everyone over the age of 12.
Moderna: Everyone over the age of 18.
Going forward, CDC will shift away from naming boosters dose 3, 4, 5, etc. From now on, everyone needs the primary series (with the original formula) and bivalent booster. This will equate to being “up-to-date” with the vaccine.
The puck now goes to Dr. Walensky at the CDC to sign off. I expect she will do this ASAP. This vaccine should be available to you very soon, depending on the timeliness of delivery to pharmacies and doctors offices.
Bottom line
Everyone aged 12+ years old should get a booster this fall. It is safe. And we are more confident than not that it will be better than the previous vaccine. It will not be the silver bullet, but will no doubt help greatly on an individual and population level through the winter months. Protect yourself and those around you.
Love, YLE
P.S. Everyone should get a booster. But, we can be data driven on when to get this vaccine based on vaccination status, age, previous infections, etc. I will write a post for tomorrow to explain my thinking. Too much for one day. Stay tuned.
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank, and at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members.






Thank you for pointing out that the risk of myocarditis from infection is higher than from vaccination. I feel like that point was missed in many stories about vaccination side effects.
Why still require 2 original formulation vaccines before the omicron boost? Why not give an omicron series followed by an omicron boost? (admittedly, there are probably not many who are not vaxed who would start now, but the rule seems to be start with the old series.) If the vaccines are only a few amino acids different, why delay for months omicron coverage for the (sadly) few new converts?