As epidemiologists, “primary prevention” is our main goal—reduce morbidity and mortality by preventing large populations from getting disease altogether. Vaccines are our best (but not only) tool to prevent severe disease from SARS-CoV-2. But even if someone gets the vaccine, there is still a small chance they could end up in the hospital. (This is especially true for those over 65 years or with comorbidities). Some immunocompromised vaccinated people are also not protected. And of course millions are not vaccinated. So, if someone gets severe disease, how can we help them?
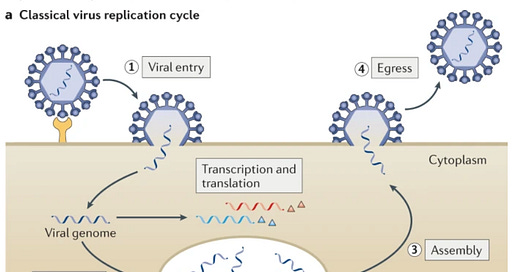
One viable option is antiviral drugs. This is a class of prescription medications that can fight a virus once someone is infected. There are many mechanisms through which antivirals can potentially help. As depicted in the figure below, once the virus enters the human body, it searches for a host cell. Then the virus’s life cycle begins: 1) entry into our cells; 2) replication within our cells; 3) assembly; and 4) escape to go infect other cells. Scientists try to create antivirals to disrupt any one of these steps.
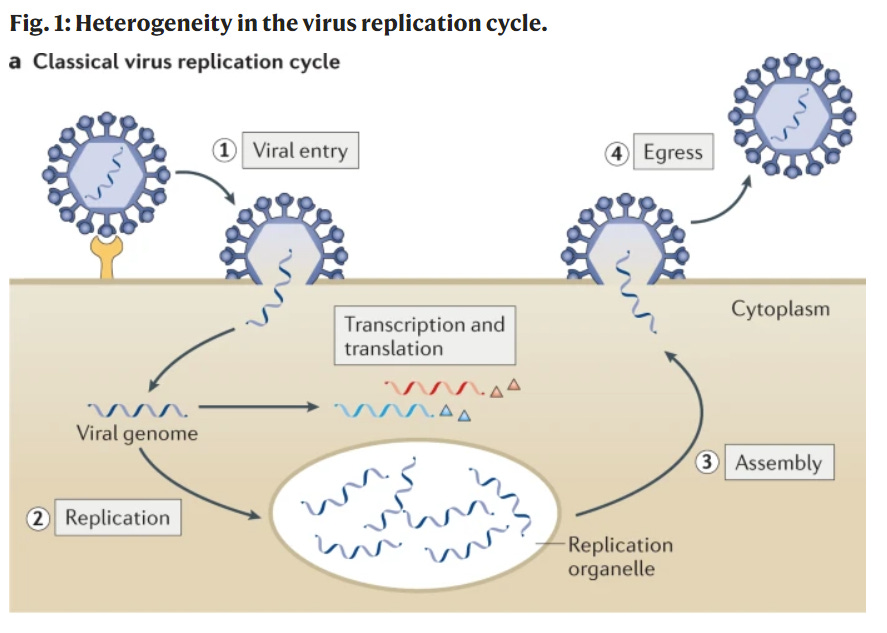
For example, oseltamivir (Tamiflu), the antiviral for the influenza, disrupts the last stage: it stops the virus from dissolving its way out so it can’t go infect others. This helps people recover from the flu 1 or 2 days earlier. But, antivirals are really difficult for scientists to make for a myriad of reasons (see my previous post here). This is why it’s taken so long to get an evidence-based antiviral, compared to, for example, vaccines, of which we now have 19 authorized and 9 approved worldwide.
Thankfully, the science for SARS-CoV-2 antivirals is finally coming through. In the past week, the FDA has authorized two for use in the United States. And this is a big deal.
Here’s what they are, what they do specifically, and pros/cons of each.
Paxlovid
This antiviral was created by Pfizer and is the most promising option so far. This treatment combines three pills that must be taken twice a day for five days. Two of the three pills are Paxlovid and the other pill is Ritonavir—a low dose HIV drug that will help the Paxlovid drug remain active in the body longer.
Paxlovid’s main mechanism is to slow down viral replication. (Stage 2 in the figure above). It does this by inhibiting one of the virus’s tools—called an enzyme—that it uses to replicate itself.
After Pfizer developed this drug, it went through randomized control trials just like vaccines do. Pfizer’s Phase III randomized clinical trial (called EPIC-HR) had 2,246 participants with a confirmed COVID19 infection that were randomized to get a five-day course of Paxlovid or a five-day course of a placebo. For this trial, all participants had to be “high risk:” unvaccinated with at least one high risk characteristic, like over the age of 65 or a comorbidity. The participants were followed for 28 days after entering the trial to see if they were hospitalized or died from COVID19. What did scientists find?
There was an 88% reduction in hospitalization and death among the Paxlovid group compared to placebo. Which is really high! Specifically,
Importantly, efficacy was similar whether the treatment was given within three or five days of symptom onset: 89% efficacy in the first three days and 88% efficacy in the first five days.
This was a huge win, because antivirals only work if given early. In the “real world,” this is difficult because it means the patient needs to realize their symptoms (which takes a few days), get tested (which is really hard right now), go to the doctor to get a prescription, and then start treatment. All of this needs to happen within a small window. The bigger that window, the better.
After receiving the full results, the FDA quickly authorized Paxlovid. They did this without convening their external scientific committee board for a review of the evidence. To me, this is a sign that the results were solid and/or an indication of the dire need for a highly effective antiviral in the wake of Omicron.
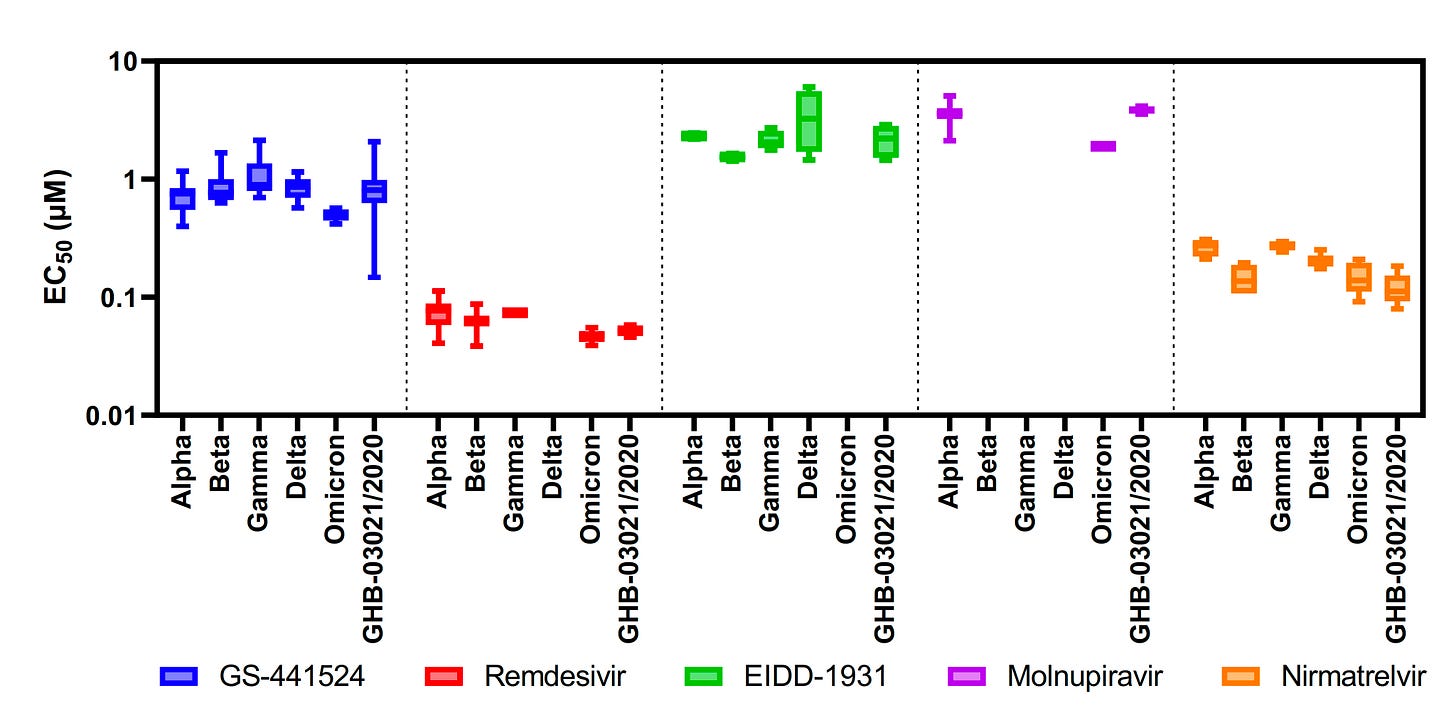
It was also very reassuring to see an independent study (i.e. science not conducted by Pfizer) come out this week confirming Pfizer’s results. The scientific group also found that this drug works against Omicron. We hypothesized that it would, because Omicron’s mutations don’t target the process mentioned above, but this is great news nonetheless. The figure below shows the effectiveness of the pill series (called Nirmatrelvir; orange lines) did not change in light of various variants of concern.
Pfizer also has two other concurrent studies using this drug:
EPIC-SR: This clinical trial evaluates the efficacy of Paxlovid among 662 people that were not considered high risk. Preliminary data showed that 2 of 333 (0.6%) patients who received Paxlovid were hospitalized compared to 8 of 329 (2.4%) who received the placebo. Unfortunately, the 0.6% isn’t statistically different than 2.4%, which means the drug isn’t too much help for non-high risk groups. But only 45% of the data is in thus far; we will see if the scale tips as more data comes in.
Concurrently, Pfizer is evaluating if, and by how much, Paxlovid blocks transmission in households. We should expect results in the first half of 2022.
And while this is all great news, the big concern is supply. Pfizer only manufactured enough pills for 65,000 people this month. When divided by 50 states, the availability gets smaller and smaller. For example, in DC—the place hardest hit with Omicron right now—there is only enough supply for 120 people. Antivirals are difficult to manufacture, so supply will ramp up slowly. Pfizer said they will have enough for 300,000 Americans by the end of February and, eventually, 120 million courses in 2022. The antiviral’s promise is also dependent on affordability and the assumption that individuals can get it in a timely manner (symptoms, test, doctor’s appointment, prescription), which is becoming more and more difficult across the country right now. There’s no doubt this antiviral will help down the road, but the promise for right now is limited.
Molnupiravir
The second antiviral drug that was authorized this week was created by Merck and and Ridgeback Biotherapeutics. It, too, is a pill series: four capsules twice a day for five days early in infection.
Molnupiravir works differently from Paxlovid, in that it does not directly slow down the replication or copying process. Instead, the drug interferes and inserts numerous mutations when the virus is replicating. As a result, the copied virus is weaker and the immune system is able to clear it much more quickly.
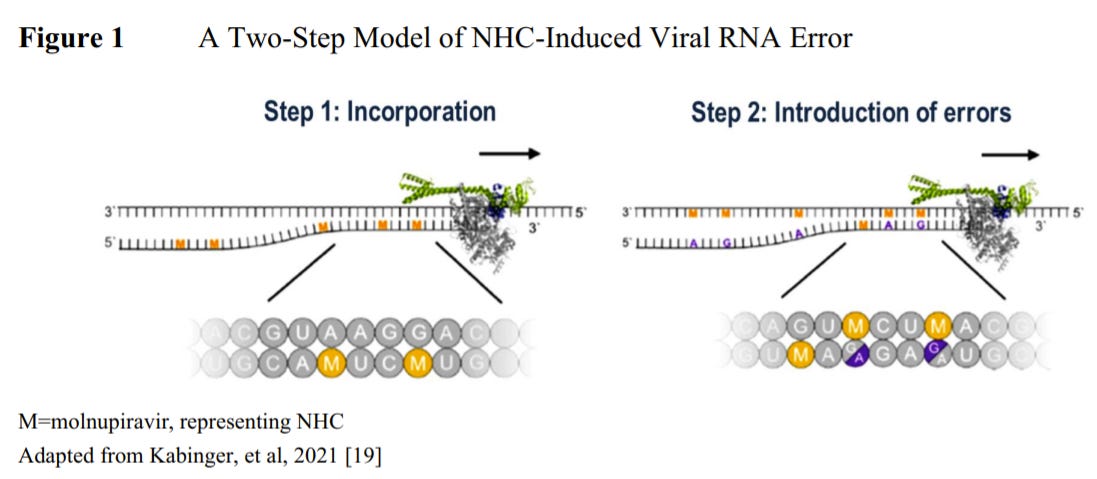
Merck’s clinical trial (called the MOVe-OUT study) had 1,433 participants with confirmed COVID19 infection that were randomized to receive a drug or placebo series between May and October 2021. After the placebo or series, the participants were followed for 29 days to see if they were hospitalized or died. This cohort of participants will also continue to be followed until their late-follow up visit at 7 months. What did Merck find? Well, this data story is fascinating:
When Merck originally submitted their FDA application they announced promising interim results: 50% reduction in hospitalization and death.
Molnupiravir group: 7.3% (28 out of 385 people) were hospitalized. No deaths were reported.
Placebo group: 14.1% (53 out of 377 people) were either hospitalized or died. Eight deaths were reported.
Importantly this analysis was only among 762 participants from May to July 2021. Once Merck analyzed data from the second half of the study in August and early October (646 additional people), they only found only a 3% reduction in hospitalization and death. This was a shocking difference. (I’m giving them the benefit of the doubt that this was truly a weird data phenomenon, rather than purposeful and deceitful).
So, pooled together (50% efficacy from first half and 3% efficacy from second half), Molnupiravir had an overall 30% reduction in hospitalization and death compared to the placebo. Results are shown below, from Merck’s presentation to the FDA.
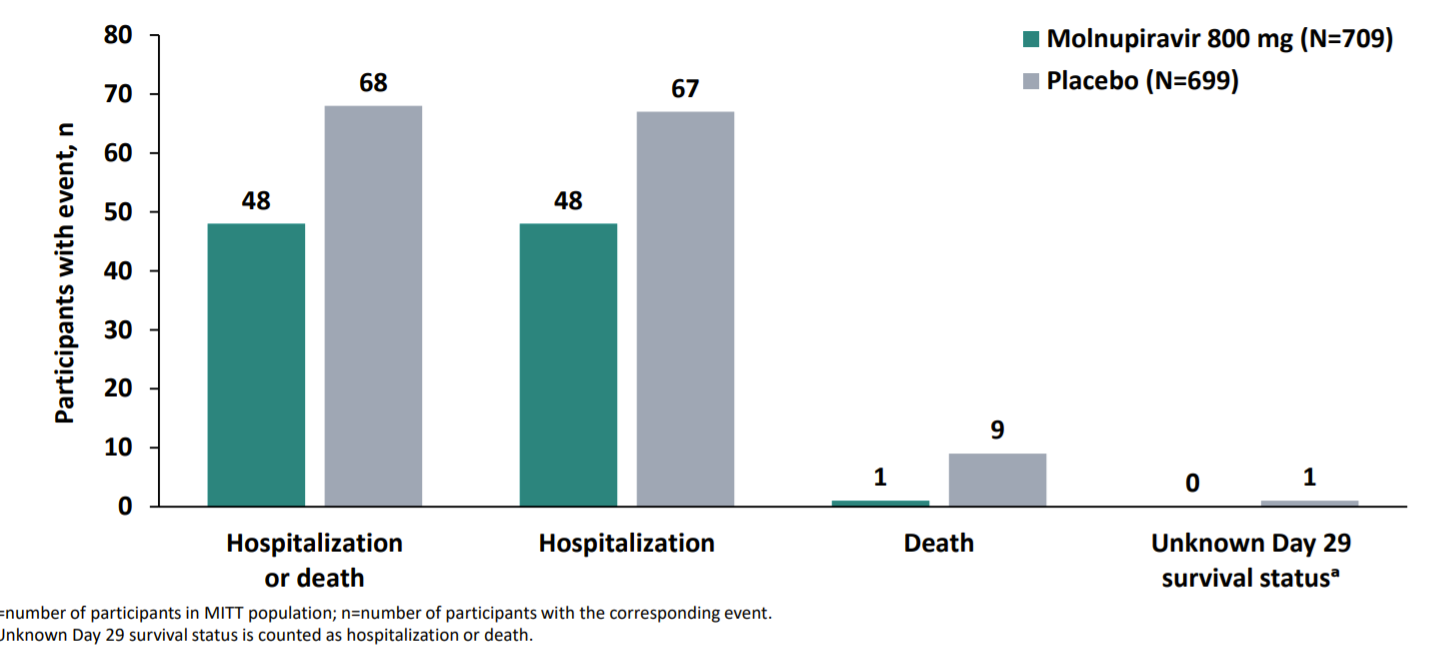
On November 30, 2021, the FDA’s external scientific advisory board met to discuss Merck’s authorization application. There were two streams of significant reservations during the discussion:
Modest efficacy: There was disappointment voiced around Molnupiravir’s modest effectiveness. The advisory board also had a lot of questions for Merck about the drastic effectiveness difference (50% to 3%). Merck didn’t have an answer for them.
Possible dangers: There was also a lot of interesting discussion about theoretical dangers. The drug works by causing of a lot of mutations in the virus (making it weaker and allowing the immune system to clear it). However, there is a risk that the virus mutates a lot but isn’t cleared. Thus, there is the theoretical danger that the drug could drive viral evolution. There is also the opportunity for the drug to change a person’s DNA. While no data has supported this, data has found this happens with pregnant rats. Some voting members voiced theoretical concerns that the drug could lead to cancer-causing mutations down the road.
This interesting discussion resulted in the external scientific committee narrowly voting to authorize this medication in a 13-10 vote. It took the FDA an abnormally long time to then take the advisory board’s vote to authorization. I was actually very surprised that the FDA ended up authorizing; I didn’t think this would happen.
Bottom line: While it may take a few months for supply, an antiviral like Paxlovid will be a game changer. I’m incredibly excited to see the science’s progression, as we can use all the help we can get in this pandemic.
Love, YLE
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD— an epidemiologist, biostatistician, professor, researcher, wife, and mom of two little girls. During the day she has a research lab and teaches graduate-level courses, but at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions, rather than decisions based in fear. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here:



More great info...
FYI - i was had covid - was double vaccinated - just finished omicron.. 2 days mild symptoms, all better now.
Previous COVID didn't help nor did 2 shots . The concept of super immunity can be put to rest... now i have 2 bouts of COVID - 2 shots.
Happy Healthy New Year to all!!!! 2022 will be an amazing year for us all.
Thank you for all you do, Katelyn!