Yesterday, ACIP—CDC’s external scientific advisory board—unanimously voted to authorize the Novavax vaccine (called NVX-CoV2373) in the U.S. This was big news for the small, underdog Maryland company who had a long road to authorization.
After rigorous clinical trials, FDA and CDC scientific meetings, and more than 1 million vaccines already rolled out across the globe, it’s clear this vaccine is safe and effective. There are two ways this vaccine could potentially help us in the U.S.:
Vaccinating the unvaccinated
Broadening protection through multiple vaccine platforms (e.g., mRNA primary series + Novavax booster)
Unfortunately, I’m not optimistic for either. I don’t think Novavax is the silver bullet we need in the U.S. to close out our fight against SARS-CoV-2.
Vaccine for the unvaccinated
Novavax was authorized as a primary series. In other words, this is intended for those who are still unvaccinated. There is still a significant need and opportunity to improve COVID-19 vaccination rates in the U.S. Around 26-37 million (10-14%) of adults have not received a COVID-19 vaccine. The majority of these folks are multiple races (30%) or non-Hispanic White (22%), living in rural areas (22%), below poverty (19%), and/or uninsured (31%).
Novavax uses a different vaccine platform than current COVID-19 vaccines do. A diverse portfolio is important for many reasons, like freeing up supply bottlenecks and providing options for those allergic to vaccines ingredients. The Novavax vaccine also may reduce vaccine hesitancy because it uses a more traditional biotechnology. Its method has a much longer track record than the newer approaches, as it’s used for some flu and HPV vaccines.
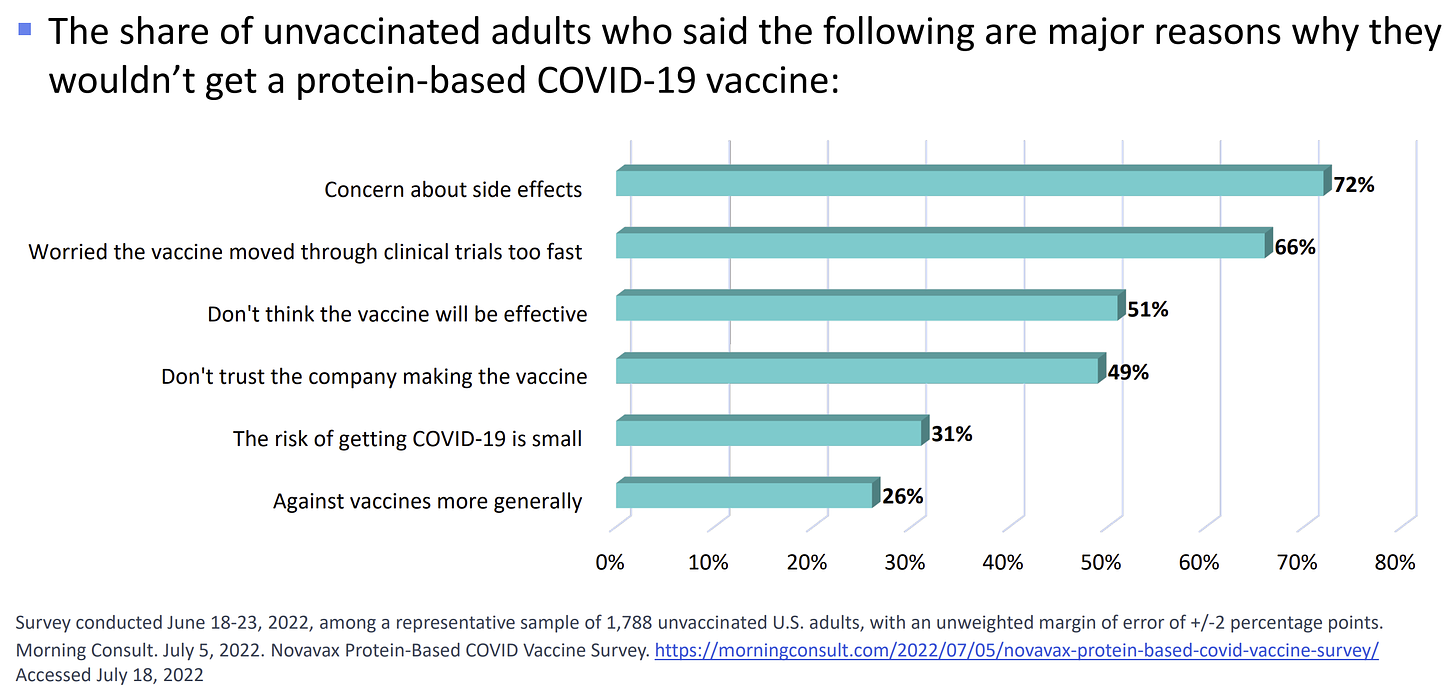
The CDC conducted a poll with unvaccinated people in the beginning of the year and found 16% of the unvaccinated would “probably” or “definitely” get the Novavax vaccine. Given pandemic fatigue and lots of infections since, it’s not surprising that this number is lower now: a more recent poll (June 2022) found only about 10% of unvaccinated people will line up for this vaccine.
Among unvaccinated people who do not want Novavax, top concerns include “concern about side effects,” “worried the vaccine moved through clinical trials too fast,” “don’t think the vaccine will be effective,” and “don’t trust the company making the vaccine.” Moving the dial is going to be difficult because it requires building trust. This isn’t done overnight with a new vaccine release.
Boosting with Novavax
The other potential is for the larger population vaccinated with mRNA vaccines to use Novavax as a booster. I was most excited about this. Originally, we hypothesized combining vaccines from different platforms would broaden protection, as the body was exposed to the virus in different ways, potentially giving it more tools. While boosting with Novavax has been shown to be safe, preliminary effectiveness of this approach is underwhelming. (To be clear, the current authorization does not “allow” Novavax boosting yet.)
One study published in Lancet assessed the effectiveness of vaccinating with one dose of mRNA with a second dose of Novavax. The figure below (left panel) shows antibodies after Pfizer+Novavax (purple) wasn’t as high as the two mRNA vaccines (orange and yellow). The same pattern was found for T-cell protection (right panel below).
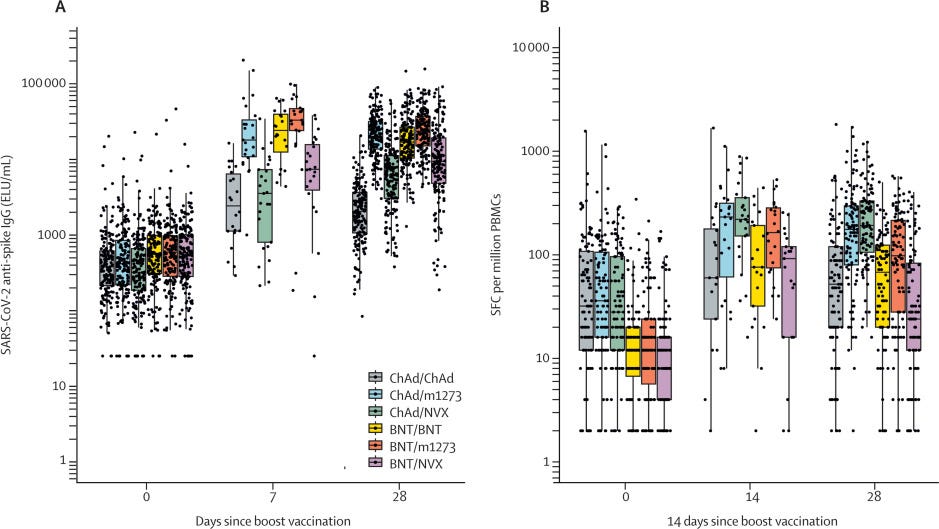
Another study in Science compared three mRNA doses with two Novavax plus one mRNA dose. The antibody response was about the same, even against some of the newer Omicron subvariants, like BA.4/5.
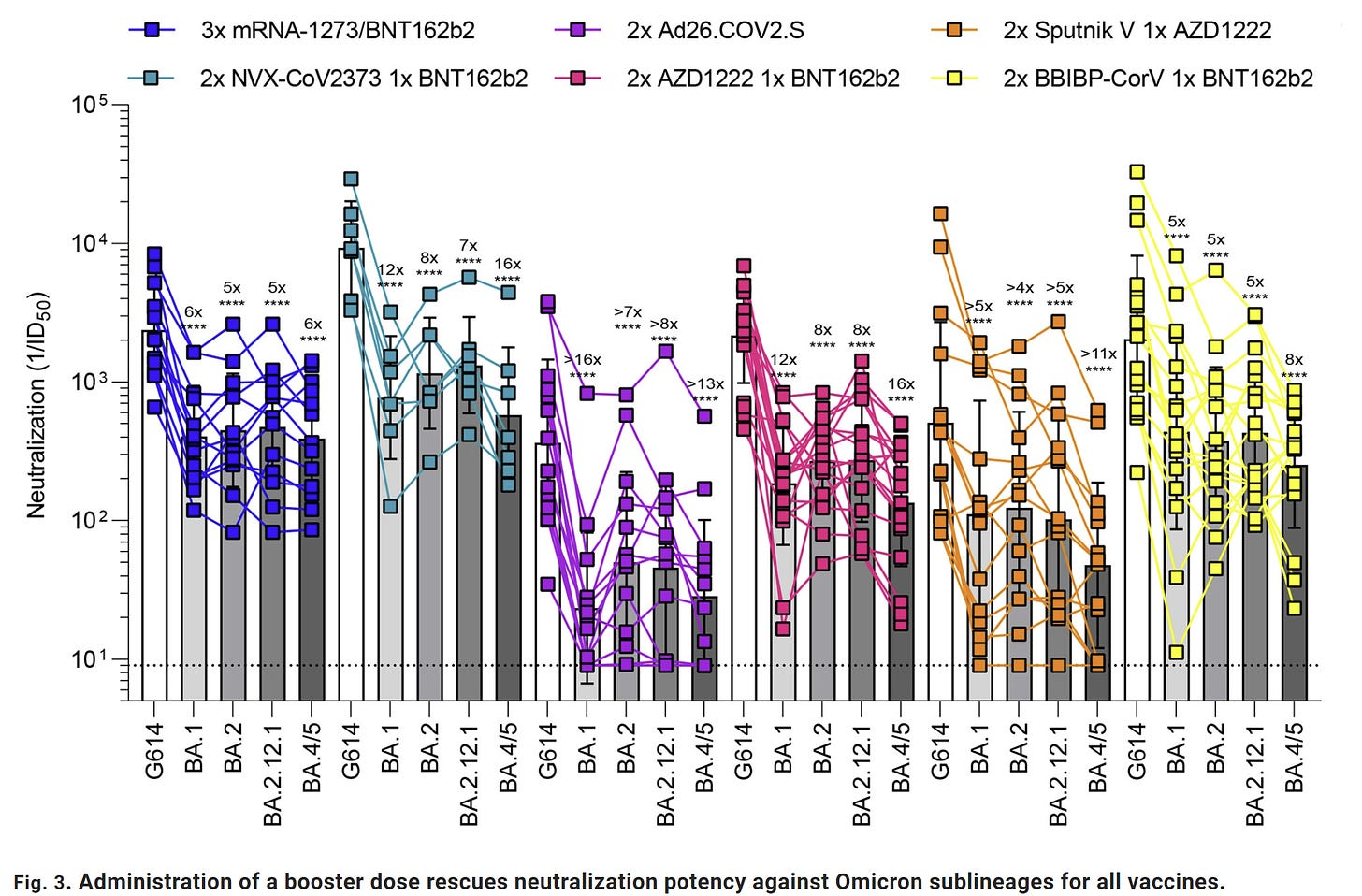
If we switch the series order (2 mRNA+1 Novavax), the story doesn’t change. A randomized control trial published in Lancet found Novavax did boost immunity, but not significantly more than other combinations. Interestingly, T-cell-boosting effects of Novavax were lower in people who had received two mRNA vaccines compared to those who received two doses of the Oxford’s Adnovirus vaccine. This study shows that combining can be advantageous for certain vaccines, but that doesn’t seem to be the case for mRNA and Novavax.
One thing we don’t know is the durability of combining mRNA with Novavax. Although antibodies may be the same, the combination could theoretically slow the rapid waning we see with the mRNA series. I’ll be anxiously awaiting the data.
Bottom line
Novavax is a great vaccine for the unvaccinated, and it will save millions of lives. It can eventually be used as a booster, but the effect will not be as impactful as I would have hoped. For now, I don’t recommend using Novavax off-label as a booster or, worse, waiting for a booster because Novavax is coming.
It’s abundantly clear that first generation vaccines, like Novavax, are not the silver bullet we need to close out this pandemic. We desperately need the funding and the drive for second generation vaccines, like pan-coronavirus vaccines (variant proof) or nasal vaccines (future post to come), to finally get ahead of this virus.
Love, YLE
In case you missed it:
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, wife, and mom of two little girls. During the day she works at a nonpartisan health policy think tank, and at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well equipped to make evidence-based decisions. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here:



Readable epidemiology! What a great concept. Thanks!
Thank you for addressing Novavax. At this point, I think an additional 10% of the unvaxxed is a win and we need those anywhere we can get them. I also think uptake would depend highly on the messaging (and that's where we will likely fail ... again). For me (vaxxed 2x and boosted 2x with mRNAs), the hope lies in Novavax having more longevity in protection than the others. Hoping that gets clarity soon.