It’s been more than 8 months since 16-17 year olds have been vaccinated in the “real world” (i.e. after clinical trials) and more than 2 months since 12-15 year olds have been vaccinated in the real world. This is a critical time period because in the history of vaccines side effects have only ever popped up 8 weeks post vaccination.
According to the American Academy of Pediatrics and the CDC, 7.1 million adolescents are fully vaccinated: 40% of 16-17 year-olds and 28% of 12-15 year-olds. This is not nearly enough adolescents protected for the new school year.
How do we increase vaccine acceptance? The biggest concern among unvaccinated adolescents (or, in reality, parents) are side effects.
Last week, the CDC published an update on the safety of vaccines among adolescents. Specifically, scientists published data from two surveillance systems: V-safe and VAERS.
V-Safe Results
V-safe is an active surveillance program in which the CDC proactively texts people to check up on othem after vaccination. (If you haven’t done so already, register here). If someone reports a concerning or severe side effect, public health workers call that person to gather more contextual information.
Since December, 129,059 adolescents enrolled in the v-safe program (66,350 aged 16–17; 62,709 aged 12-15). What are the numbers telling us?
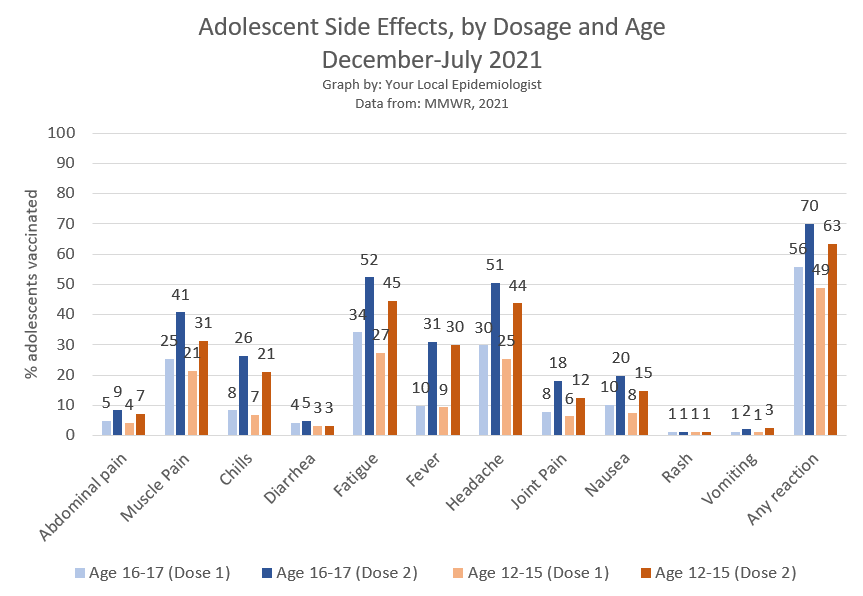
49-56% of adolescents experience a side effect after dose 1. 63-70% report side effects after dose 2.
24% of adolescents were unable to perform normal daily activities the day after dose 2.
The most commonly reported side effect was fatigue, followed by headaches, muscle pain, and fever.
0.5-0.8% of adolescents required medical care in the week after receipt of either dose
0.02-0.04% adolescents were hospitalized (although, v-safe does not record reason for hospitalization, so we don’t know if it was related to the vaccine or not)
VAERS Results
The publication also highlighted VAERS data. This is a passive surveillance program in which people (or more commonly physicians) voluntarily report symptoms. Liked I’ve said before, take VAERS with a grain of salt. Anyone can post anything to this site leading to a number of biases. But, nonetheless, it’s been a useful tool to use throughout the pandemic to find safety signals.
From December to July 2021, there were 9,246 reports of adverse events for adolescents aged 12–17 years after vaccination.
90.7% of the reports were for non-serious events like dizziness (20.1%), headache (11.1%), and fainting (6%; majority due to anxiety of needles)
9.3% of the reports were for serious events, like chest pain (56.4%). Myocarditis was listed among 4.3% (397) of all reports
CDC investigated 14 reports of death after vaccination, none of which have been causally linked to the vaccine. Cause of deaths were pulmonary embolism (2), suicide (2), head injury (2), heart failure (1), and bacterial infection (1). Six deaths are currently under investigation.
And, just because I know people will ask…
What are the latest myocarditis numbers? Other than the VAERS data above, the latest data we (the public) have on myocarditis is from the June 11, 2021 at an ACIP meeting. For adolescents aged 12-17 years old, there were 147 cases of myocarditis investigated by the CDC. I posted about this meeting is great detail (see here). This rate was higher among males compared to females:
Females: 8 myocarditis cases per 1M. As a reminder, we expect 2 per 1M to happen in the background.
Males: 63 myocarditis cases per 1M. As a reminder, we expect 4 per 1M to happen in the background. Observed is much higher than expected, signaling a link to vaccines
No deaths linked to myocarditis
The CDC continues to review the data in real time to ensure a favorable risk-benefit ratio. You can read more about this on the CDC website here. The benefits of the vaccine continue to greatly outweigh the risks of myocarditis (or COVID-19 disease).
Where are the Moderna results for adolescents? The last I heard, Moderna submitted their EUA application early June. We should be hearing soon I’m not sure why it’s taking longer than expected. But remember, Pfizer took longer with adolescents than adults though too, so we just have to remain patient. This is not a sign that the mRNA vaccine is not safe for adolescents.
When will vaccines for under 12 be available? I’m still hopeful for late in the year. Vaccine sponsors are testing smaller doses for kids, so they had to start from square one (i.e., Phase I). Phase I is complete and Phase II/III is well on it’s way. Pfizer will have data by end of September. Word on the street is that they will be going for full approval (rather than emergency authorization). So this process will take longer. I don’t think anything will make the FDA rush this process. They need to get it right to ensure confidence in the vaccines.
Bottom Line: We found the myocarditis safety signal within one month of 12-15 years olds vaccinated. Which means our surveillance systems are working fantastically. Even with this rare side effect, the vaccine continues to be safe among adolescents and the benefits greatly outweigh risks.
Love, YLE
P.S. With this I updated the fact sheet for adolescent vaccines. If you’re a paid subscriber, let me know if you want an updated PDF






Is there any good data on the myocarditis(or any other complication) rates with a covid infection? Particularly with kids. My gut it is significantly higher than the vaccine by how much was picked up on return to play evaluations for athletes.
I am a pediatrician and I would love a pdf copy of the chart to use in my office - its fantastic!!!